Abstract
Mycobacterium avium ss. paratuberculosis (MAP) is the cause of Johne’s disease, an enteric inflammatory disease mostly studied in ruminant
animals. MAP is also the putative cause of the very similar human malady, Crohn’s disease. Recently, MAP has been associated with additional
human inflammatory/autoimmune diseases: autoimmune thyroiditis, autoimmune diabetes, Blau syndrome and multiple sclerosis. The etiology
of autoimmunity is multi-factorial with both genetically determined risk factors and environmental triggers. Systemic lupus erythematosus (SLE)
and Sjogren’s syndrome (SS) are autoimmune diseases that are related both clinically and immunologically which may simultaneously occur
in the same individual. Both diseases are associated with systemic autoimmunity frequently presenting with antinuclear antibodies (ANA). Two
of these autoantigens, common immune targets in both SLE and SS, are the Ro (or SSA) and La (or SSB) ribonucleoproteins. Auto-antibodies
to these proteins are frequently found in the sera of individuals with SLE and SS. It has been suggested that MAP triggers autoantibodies via
mimicry between protein elements of its immunodominant heat shock protein, HSP65, and host tissue proteins. Animal studies support a role
for mycobacterial HSP65 in SLE. This article presents the case of a child with SLE and SS. She has both the anti-Ro and anti-La antibodies and
MAP was cultured from her blood. BLAST analysis of MAP HSP65 showed homology with the anti-Ro and anti-La proteins. Further investigation
of a link between MAP and SLE/SS is warranted.
Background
Lupus and Sjogren’s
Systemic lupus erythematosus (SLE) and Sjogren’s syndrome (SS) are
autoimmune diseases that are related both clinically and immunologically.
SLE is a clinically diverse chronic inflammatory disease resulting from
the interplay of genetic, hormonal, and environmental factors. [1] SS is
a chronic inflammatory and autoimmune disorder that is characterized
by diminished lacrimal and salivary gland secretion resulting in
keratoconjunctivitis sicca (dry eye) and xerostomia (dry mouth) [2,3].
The two diseases can occur together in the same person. Where this
occurs, SS is usually considered secondary to the occurrence of SLE, as it
is with other autoimmune diseases such as rheumatoid arthritis. There is
no known cause for either SLE or SS.
SLE and SS are associated with systemic autoimmunity frequently in
the form of antinuclear antibodies (ANA) [3]. The individual self proteins
targeted by ANA have been elucidated and characterized. One of these
auto-antigens, a common target of autoimmunity in both SLE and SS, is
the Ro (aka SSA) ribonucleoprotein [5].
Anti-Ro is found in the sera of up to 50% of SLE patients and a
higher percentage of patients with SS [5]. Originally identified by double
immunodiffusion in the sera of SLE patients with no ANA [6], and later
identified as SS-A in the sera of SS patients [7], anti-Ro is associated with
several clinical aspects of both diseases including lymphopenia, leukopenia
and hypergammaglobulinemia [8]. Anti-La (aka SSB) autoantibodies are
found in sera from patients with SLE or SS, but are invariably found in
sera that also contain anti Ro. Anti-Ro without anti-La is more commonly
found in patients with SLE, while anti-Ro along with anti-La is more
common in SS [9].
MAP
Mycobacterium avium ss. paratuberculosis (MAP) is a Gram-positive,
acid-fast staining small rod-shaped bacterium. MAP causes a chronic
granulomatous inflammation of the intestines in ruminant animals called
Johne’s disease; mostly studied in dairy cattle, goats, and sheep. MAP also
causes a chronic inflammation of the intestines in beef cattle and in a wide
variety of other domestic and wild ruminants. A majority of the dairy
herds in the United States and Europe have MAP infected animals within
the herd [10].
MAP and human exposure
Infected cows shed up to 1.6×107
organisms per 2 grams of manure
(0.07 oz) – a dose large enough to infect a calf. A single high-shedding
animal can excrete up to 15 gallons of such contaminated manure per
day - a staggering 25,000 infective doses per day [11]. MAP is present
in pasteurized milk [12,13], infant formula made from pasteurized milk
[14], surface water [15,16], soil [15], cow manure ‘‘lagoons’’ that leach into
surface water, cowm manure in both solid and liquid forms that is applied
as fertilizer to agricultural land [17,18], and municipal tap water [18,19].
This provides multiple routes of transmission to humans.
Case Report
An 11-year old female with cutaneous lupus erythematosus (biopsy
proven) and Sjogren’s syndrome was seen. She had a history of facial
malar rash, several bouts of parotitis as well as leukopenia. She had no
sign of renal involvement. Significant laboratory tests included positive
ANA and antibodies to Ro and La. Peripheral blood was sent for culture
for Mycobacterium avium ss. paratuberculosis (Figure 1), the culture was
positive after six months as identified by IS900, indicative of MAP*. Blast
analysis of both Ro and La antibodies showed positively with epitopes of
MAP HSP65 (Figure 2).
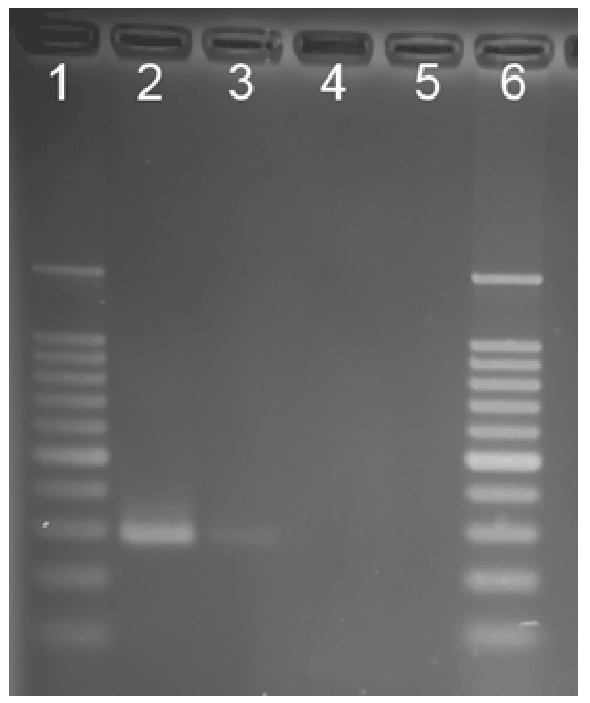
Figure 1: Nested IS900 PCR products. Lane 1, molecular weight
standards ladder; Lane 2, M. a. paratuberculosis positive control; Lane
3, patient sample; Lane 4, sample negative control; Lane 5, PCR reagent
negative control, Lane 6, molecular weight standards ladder
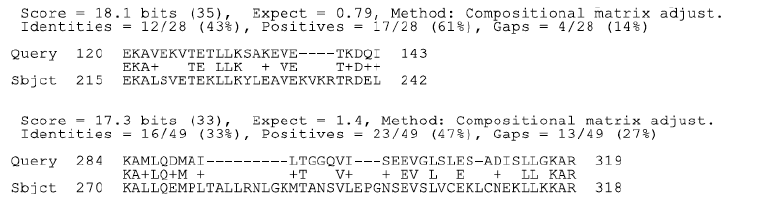
Figure 2a: BLAST analysis HSP65 and Ro (SSA)
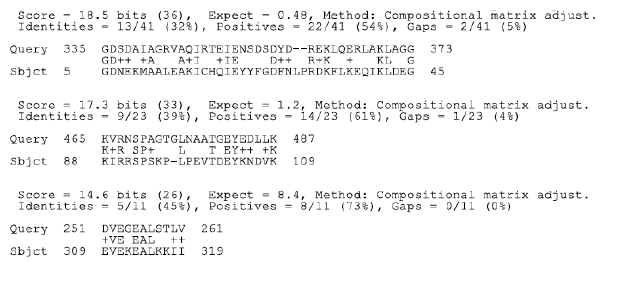
Figure 2b: BLAST analysis HSP65 and La (SSB)
Discussion
Molecular mimicry
The concept of molecular mimicry is based on a structural similarity
between a pathogen and self. The similarity could be expressed as shared
amino acid sequences or similar conformational structure between a
pathogen and self antigens. Molecular mimicry has become a very popular
explanation for the frequent association of infection with autoimmune
disease [20].
Heat shock proteins are found in virtually all life forms and are
closely linked to the immune response. HSP65 of mycobacteria is an
immunodominant antigen. In human mycobacterial infection it has been
estimated that up to 40% of the T-cell response is directed against this
single protein [21,22]. The HSP65 of MAP was compared to amino acid
sequences of the Ro and La proteins. It is postulated that the similarity
manifest between HSP65 and Ro and La is the trigger of anti-Ro and antiLa
antibodies (Figure 2). The HSP65 of M. leprae significantly accelerates
the progression of SLE in a standard murine model of SLE [23].
MAP and human granulomatous disease: Crohn’s and
Sarcoidosis
In addition to Johne’s disease of animals, MAP is the putative cause
of the very similar Crohn’s disease of humans. The DNA of MAP can
be identified within the granulomas of Crohn’s biopsies [24] and, with
extreme care and patience, MAP can be grown from the gut and blood
of Crohn’s patients [25-27]. In limited series, anti-mycobacterial therapy
directed at MAP has been shown to have a favorable effect on patients
with Crohn’s disease [28]. Moreover, MAP has been historically linked
is sarcoidosis; a multisystem inflammatory disease in which DNA
evidence of MAP has been found (sporadically) in sarcoid granulomas
[29]. Juvenile sarcoidosis (Blau syndrome) is an inherited granulomatous
disease of children. The DNA of MAP was detected from every sample in
a small series of archived tissues [30].
MAP and type 1 diabetes, autoimmune thyroiditis, and Multiple
sclerosis
While it is not difficult to envision a role for MAP in human disease
where there is a granuloma, it is more difficult to assign a role to MAP
in diseases that feature autoantibodies. This divide is bridged by the
concept that MAP HSP65 mimics host protein elements. An example is
that of MAP as a proposed infectious trigger of autoimmune diabetes.
T1DM is an autoimmune disease manifest by progressive T cell-mediated
autoimmune destruction of insulin-producing beta cells in the pancreatic
islets of Langerhans [31]. In 2005, Dow postulated a causative role for
MAP in the T1DM [32].
Sechi et al. in 2007 found the DNA of MAP in the blood of autoimmune
(type 1) patients but not nonautoimmune (type 2) diabetics [33-35].
(Sechi also found an association of polymorphisms of the SLC11a1 gene
and MAP in T1DM patients [36].) The link connecting MAP and T1DM:
MAP HSP65 mimic the host pancreatic glutamic acid decarboxylase
(GAD) [37]. Similar mechanisms are proposed for the role of MAP in
autoimmune (Hashimoto’s) thyroiditis [38, 39] and multiple sclerosis [40].
Summary
MAP is associated with an increasing number of human diseases. In this
case report MAP bacteremia was found in a child with lupus and Sjogren’s
syndrome. MAP bacteremia may trigger SLE and SS via molecular
mimicry: the persistent presence of MAP results in its production of
mycobacterial HSP65, the response to HSP65 results in the production
of antibodies to Ro and La which produce inflammation characteristic of
lupus erythematosus and Sjogren’s syndrome.
Acknowledgements
The author acknowledges, with gratitude, the expertice of Dr. Mike
Collins and his laboratory at the University of Wisconsin-Madison for his
testing of the sample from this patient.
Download Provisional PDF Here
Article Information
Article Type: Case Report
Citation: Dow CT (2016) Detection of M.
paratuberculosis Bacteremia in a Child With
Lupus Erythematosus and Sjogren’s Syndrome.
Autoimmun Infec Dis 2(1): doi http://dx.doi.
org/10.16966/2470-1025.111
Copyright: © 2016 Dow CT. This is an open-access
article distributed under the terms of the Creative
Commons Attribution License, which permits
unrestricted use, distribution, and reproduction
in any medium, provided the original author and
source are credited.
Publication history:
Received date: 28 Sep 2015
Accepted date: 13
Jan 2016
Published date: 25 Jan 2016