Introduction
Public perception of Cannabidiol (CBD) is overwhelmingly favorable due to the plethora of supposed health benefits, including analgesic, anxiolytic, and anti-inflammatory effects [1]. However, many of these claims have yet to be researched, and potential health and safety risks have not been thoroughly investigated. One approach to assessing health status is the use of mass spectrometry-based metabolomics, which can analyze metabolic changes in response to diet, disease, or other factors [2,3].
Two metabolomics methods are available: targeted metabolomics, which quantifies defined groups of metabolites, and untargeted metabolomics, which provides a comprehensive analysis of all measurable analytes in a sample, including unknowns. In recent years, Chemical Isotope Labeling (CIL) and Liquid ChromatographyMass Spectrometry (LC-MS)-based untargeted metabolomics has provided an opportunity to analyze metabolites based on chemical groups, including metabolites containing carboxyl groups, including fatty acids and their derivatives, and hydroxyl groups, which includes important biological compounds like hormones [4,5]. If there are no known metabolites of interest, untargeted metabolomics can also be used to identify specific biomarkers that can then be used in targeted metabolomics or pathway analyses in future work.
In our previous study, we applied a CIL/LC-MS-based untargeted metabolomics technique to examine differences in plasma metabolites containing amine/phenol and carbonyl chemical groups in dogs receiving CBD-containing treats compared with control [6]. However, CBD has been suggested to alter other metabolic processes, like lipid metabolism, that are not represented by those labeling methods [7]. Therefore, the objective of the current, exploratory study was to evaluate the impact of oral CBD supplementation on the canine carboxyl and hydroxyl submetabolomes using untargeted metabolomics and biomarker analysis. We hypothesized that after 3 weeks of supplementation, CBD would alter the canine carboxyl- and hydroxyl-metabolome compared with control.
Materials and Methods
This study was approved by the Lincoln Memorial University (LMU) institutional animal care and use committee (protocol 1911- RES) before the start of the study. All housing and husbandry were provided in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (8th edition.), and all applicable LMU protocols.
Subjects and diets
Sixteen dogs (8 male, 8 female, 9 months to 4 years of age, 18.2 ± 3.4 kg BW) of various mixed breeds were received from a local shelter for inclusion in this study. The shelter was informed of and gave consent for the use of the dogs for research purposes before their arrival. Before beginning the experiment, each dog had a Complete Blood Count (CBC), and serum chemistry analysis (IDEXX Laboratories, Inc., Westbrook, Maine) performed, along with a physical evaluation by the attending veterinarian and a fecal examination to rule out any underlying disease that might preclude enrollment. Dogs were excluded if they demonstrated serious behavioral issues, such as aggression that would endanger research personnel, were severely emaciated, classified as a body condition score < 2 on a 5-point scale (where 1 is emaciated and 5 is obese), or if their initial evaluations revealed an underlying disease that required more than routine treatments (i.e. heartworm positive test result).
Dogs were individually housed in 1.2 × 1.8 m kennels within one of two dog wards at the LMU DeBusk Veterinary Teaching Center. They were stratified by treatment and sex and evenly distributed between the two wards. Dogs were fed Purina Pro Plan EN Gastroenteric Fiber Balance Dry Dog Food (Nestle Purina, Inc., St. Louis, MO) to meet the daily metabolizable energy requirements of neutered adult dogs at maintenance, calculated as (70* BW0.75) * 1.6 and split into two meals per day. Body weight and body condition score (5-point scale) were assessed weekly for the adjustment of diets. Dogs arrived from the shelter and started on the study diet more than 37 days prior to starting treatments and 58 days before collecting samples for this study.
Experimental design and treatments
These dogs were participating in a concurrent study [8] evaluating the impact of CBD on canine voluntary activity with treatments consisting of 0 (placebo treats; CON) and 4 mg CBD/kg BW/d (CBD). Dogs were blocked by baseline activity before being stratified by age, weight, and sex and randomly assigned to treatments within each block. The CBD was a constituent of a proprietary industrial hemp extract (AgTech Scientific, Paris, KY) that was incorporated into treats and administered in the form of 1 treat twice daily, each containing half the daily dose. Both CON and CBD treats were composed of the following ingredients: chicken, chicken liver, Asian carp, catfish, and in the case of the CBD treats, industrial hemp extract. Treats were offered solely as a reward upon kennel re-entry following twice-daily exercise within 30 min of meals.
Blood sample collection
As previously described [6], 6 mL of blood was collected via cephalic catheter or jugular venipuncture after 21 d of treatment administration approximately 2 hours after the final treat administration. Blood samples were collected into tubes containing sodium heparin and were immediately centrifuged at 1645 x g for 10 min. Plasma was collected after centrifugation then stored at -20°C (<12 hours) before long-term storage at -80°C.
CIL/LC-MS-based untargeted metabolomics analysis
As previously described [6], untargeted metabolomic profiling was done using a CIL/LC-MS-based technique with an Agilent 1100 LC system (Palo Alto, CA) connected to a Bruker Impact HD Quadrupole Time-Of-Flight (QTOF) MS (Billerica, MA). Detailed information regarding sample preparation, labeling, normalization, LC-UV and LC-MS setup, and metabolite quantification have been reported elsewhere. In this study, carboxyl- and hydroxyl-containing metabolites were analyzed. A total of 19 LC-MS data files were generated (3 quality control samples, 8 CBD samples, and 8 CON samples).
Metabolite data processing
Raw data processing on the 19 LC-MS data files was performed using ISOMS Pro 1.0 according to procedures described. Peak pairs whose mean (sample) / mean (blank) was ≤ 4.0 were filtered out. Peak pairs with no data present in at least 80% of the samples were filtered out. The final metabolite-intensity table was generated using IsoMSQuant.
Metabolite identification
A two-tier identification approach was used to perform metabolite identification. In tier 1, peak pairs were searched against a Chemical Isotope-Labeled Metabolite Library (CIL Library) based on accurate mass and retention time. The CIL Library contains 1, 213 experimental entries, including 271 carboxyls and 141 hydroxyls. In tier 2, a Linked Identity Library (LI Library) was used for the identification of the remaining peak pairs. The LI Library includes over 2000 human endogenous metabolites from 68 metabolic pathways, providing highconfidence putative identification results based on accurate mass and predicted retention time matches.
Statistical analysis
The final metabolite intensity tables for the carboxyl- and hydroxylcontaining metabolome were imported separately into Metabo Analyst 5.0 software package for statistical analysis. Before statistical analysis, the data were log-transformed, normalized by the median, and autoscaled. Median scaling eliminated unwanted inter-sample variations to make the individual samples more comparable to each other and auto-scaling made the metabolites more comparable in magnitude to each other.
Univariate (volcano plot) and multivariate analysis (Partial Least Squares Discriminant Analysis [PLS-DA] scores plot) were then generated to identify overall treatment differences across the multivariate dataset. The volcano plot was constructed by plotting the fold change (FC; CBD/CON) of each metabolite against its P-value. Metabolites with FC ≥ 1.2 or ≤ 0.83 having a false discovery ratio (FDR) ≤ 0.05 were considered to be differentially increased or decreased relative to CON, respectively.
The utility of the metabolites with FC ≥ 1.2 or ≥ 0.83 and FDR ≤ 0.05 to serve as potential biomarkers of the effects of CBD was tested using a Receiver Operating Characteristic (ROC) curves as calculated by the ROCCET web server using MetaboAnalyst 5.0 software package. Metabolites with an area under ROC (AUROC) ≥ 0.90 and a P ≤ 0.05 were considered excellent biomarkers.
Results
Carboxyl-metabolome
Within the carboxyl analysis, a total of 2,943 unique peak pairs were detected. Of those peak pairs, 29 peak pairs were positively identified in tier 1 (CIL Library; Supplementary Table 1) and 144 peak pairs were putatively identified with high confidence in tier 2 (LI Library; Supplementary Table 2). The PLS-DA scores plot (Figure 1A) show clear separation between CON and CBD samples, and the permutation test (P = 0.02) confirms the validity of the PLS-DA model (Supplementary Figure 1).
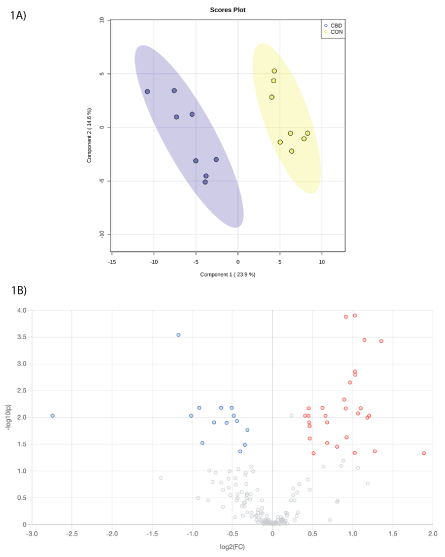
Figure 1: A. Partial least squares discriminant analysis (PLS-DA) scores plot and B. volcano plot showing the differential carboxyl-containing metabolites. Fold change (FC) ≥ 1.2 (in red) or ≤ 0.83 (in blue) with false discovery ratio (FDR) ≤ 0.05 are differentially increased or reduced by cannabidiol (CBD) relative to control (CON).
| Metabolite |
Normalized RT1 |
FC |
FDR |
Identification Level2 |
| 2,5-Dioxopentanoate |
741.4 |
3.70 |
0.047 |
Tier 2 |
| Isomer of Hydroxypropionic Acid |
380.9 |
2.56 |
<.001 |
Tier 1 |
| 4-Coumarate |
802.6 |
2.42 |
0.043 |
Tier 2 |
| Isomer of D-Glycerate |
255.0 |
2.31 |
0.009 |
Tier 2 |
| Acetic Acid |
560.5 |
2.21 |
<.001 |
Tier 1 |
| D-Glycerate |
299.4 |
2.14 |
0.007 |
Tier 2 |
| Isomer of Glycolate |
282.4 |
2.09 |
0.008 |
Tier 2 |
| Isomer of Threonate |
272.2 |
2.09 |
0.088 |
Tier 2 |
| 5-Deoxy-D-Glucuronate |
361.4 |
2.04 |
0.002 |
Tier 2 |
| Citramalic Acid |
449.1 |
2.04 |
0.001 |
Tier 1 |
| 3,4-Dihydroxymandelic Acid |
493.9 |
2.04 |
<.001 |
Tier 1 |
| Glycolate |
299.4 |
2.03 |
0.046 |
Tier 2 |
| 3-Oxopropanoate |
361.4 |
1.95 |
0.002 |
Tier 2 |
| Ethyl Malonate |
739.7 |
1.89 |
<.001 |
Tier 1 |
| Isomer of 3-Oxopropanoate |
332.4 |
1.88 |
0.007 |
Tier 2 |
| Isomer of 3-Hydroxybutyric Acid |
474.9 |
1.86 |
0.005 |
Tier 1 |
| 2-Hydroxy-3-Oxopropanoate |
389.2 |
1.81 |
0.080 |
Tier 2 |
| Hydroxyisobutyric Acid |
548.8 |
1.60 |
0.012 |
Tier 1 |
| Isovaleric Acid |
1039.8 |
1.60 |
0.030 |
Tier 1 |
| Butyric Acid |
870.3 |
1.58 |
0.009 |
Tier 1 |
| Glyoxylate |
528.3 |
1.55 |
0.083 |
Tier 2 |
| Nicotinate |
574.9 |
1.54 |
0.007 |
Tier 2 |
| 3-Hydroxybutyric Acid |
501.4 |
1.43 |
0.047 |
Tier 1 |
| S-5-Amino-3-Oxohexanoic Acid |
596.0 |
1.38 |
0.025 |
Tier 2 |
| Hydroxypropionic Acid |
424.8 |
1.38 |
0.015 |
Tier 1 |
| Lactic Acid |
452.2 |
1.37 |
0.012 |
Tier 1 |
| Isomer of Lactic Acid |
471.2 |
1.36 |
0.009 |
Tier 1 |
| Isovanillic Acid |
739.5 |
1.33 |
0.009 |
Tier 1 |
| 4-Oxoproline |
798.3 |
0.80 |
0.017 |
Tier 2 |
| 2-Aminomuconate Semialdehyde |
799.6 |
0.79 |
0.032 |
Tier 2 |
| Isomer of 9-Oxononanoic Acid |
1048.9 |
0.74 |
0.093 |
Tier 2 |
| L-1-Pyrroline-3-Hydroxy-5-Carboxylate |
372.5 |
0.74 |
0.012 |
Tier 2 |
| S-4-Amino-5-oxopentanoate |
439.3 |
0.70 |
0.007 |
Tier 2 |
| Isomer of 1-Aminocyclopropane-1-Carboxylate |
272.2 |
0.67 |
0.013 |
Tier 2 |
| L-Allothreonine |
439.3 |
0.64 |
0.007 |
Tier 2 |
| Arginine |
279.8 |
0.60 |
0.012 |
Tier 2 |
| Isomer of Aspartate |
574.6 |
0.58 |
0.098 |
Tier 2 |
| Isomer of Jasmonic Acid |
1525.6 |
0.55 |
0.030 |
Tier 2 |
| 2-Oxo-4-Phenylbutyric Acid |
1150.3 |
0.53 |
0.007 |
Tier 2 |
| 9,10-12,13-Diepoxyoctadecanoate |
1333.8 |
0.50 |
0.009 |
Tier 2 |
| Jasmonic Acid |
1437.0 |
0.44 |
<.001 |
Tier 2 |
| Isomer of 1-Pyrroline-2-Carboxylate |
1160.7 |
0.15 |
0.009 |
Tier 2 |
Table 1: Identified carboxyl-containing metabolites affected by cannabidiol (CBD) compared to control (CON). Metabolites with a fold change (FC) ≥ 1.2 relative to CON and a false discovery ratio (FDR) ≤ 0.05 considered increased in the CBD compared to CON. Metabolites with a FC ≤ 0.83 and an FDR ≤ 0.05 considered reduced in CBD compared to CON.
1Normalized RT (retention time) shows the corrected retention time of the peak pair with Universal RT Calibrant data.
2Tier 1 indicates positive metabolite identification within the chemical isotope labeling (CIL) metabolite library whereas Tier 2 indicates high confidence putative identification within the linked identity (LI) library.
Volcano plot analysis showed that 42 metabolites were differentially altered (FC ≥ 1.2 or ≤ 0.83, FDR ≤ 0.05) by CBD (Figure 1B). Twentyeight metabolites (Table 1) were differentially reduced (FC ≤ 0.83, FDR ≤ 0.05) by CBD compared to control.
Univariate analysis of the 42 carboxyl-containing metabolites positively and putatively identified that were differentially altered by CBD revealed that 23 metabolites appear to be highly predictive of the metabolomic changes between CBD and CON (AUROC ≥ 0.90; P< 0.001; Figure 2).

Figure 2: Biomarker analysis of carboxyl metabolites for CBD (CBD) and control (CON) treatments identified A. jasmonic acid (AUROC = 1.00; P < 0.001); B. (S)-4-amino-5-oxopentanoate (AUROC = 1.00; P < 0.001); C. 3,4-dihydroxymandelic acid (AUROC = 1.00; P < 0.001); D. isomer of 1-aminocyclopropane-1-carboxylate (AUROC = 1.00; P < 0.001); E. isomer of hydroxypropionic acid (AUROC = 1.00; P < 0.001); F. L-1-pyrroline-3- hydroxy-5-carboxylate (AUROC = 1.00; P < 0.001); G. L-allothreonine (AUROC = 1.00; P < 0.001); H. 2-oxo-4-phenylbutyric acid (AUROC = 0.98; P < 0.001); I. Arginine (AUROC = 0.98; P < 0.001); J. citramalic acid (AUROC = 0.97; P < 0.001); K. 4-oxoproline (AUROC = 0.95; P < 0.001); L. 5-deoxy-Dglucuronate (AUROC = 0.95; P < 0.001); M. 9,10-12,13-diepoxyoctadecanoate (AUROC = 0.95; P < 0.001); N. isomer of 1-pyrroline-2-carboxylate (AUROC = 0.95; P < 0.001); O. isomer of aspartate (AUROC = 0.95; P < 0.001); P. 2-aminomuconate semialdehyde (AUROC = 0.94; P < 0.001); Q. isomer of 9-oxononanoic acid (AUROC = 0.94; P < 0.001); R. acetic acid (AUROC = 0.92; P < 0.001); S. ethyl malonate (AUROC = 0.92; P < 0.001); T. isomer of jasmonic acid (AUROC = 0.92; P < 0.001); U. 3-oxopropanoate (AUROC = 0.91; P < 0.001); V. D-glycerate (AUROC = 0.91; P < 0.001); and W. isomer of D-glycerate (AUROC = 0.91; P < 0.001) as candidate biomarkers.
Hydroxyl-metabolome
Within the hydroxyl analysis, a total of 3,759 unique peak pairs were detected. Of those peak pairs, 141 peak pairs were positively identified in tier 1 (CIL Library; Supplementary Table 1) and 65 peak pairs were putatively identified with high confidence in tier 2 (LI Library; Supplementary Table 2). The PLS-DA scores plot (Figure 3A) show a clear separation between CON and CBD samples, and the permutation test (P< 0.01) confirms the validity of the PLS-DA model (Supplementary Figure 2).
| Metabolite |
Normalized RT1 |
FC |
FDR |
Identification Level2 |
| 17alpha,20alpha-Dihydroxypregn-4-en-3-one |
1618.8 |
6.19 |
0.001 |
Tier 2 |
| D-Tagatose |
235.4 |
2.35 |
0.001 |
Tier 2 |
| 3,4-Dihydroxyphenylpropanoate |
678.7 |
2.09 |
0.011 |
Tier 2 |
| L-Rhamnono-1,4-Lactone |
280.5 |
1.91 |
0.001 |
Tier 2 |
| L-Rhamnofuranose |
198.0 |
1.67 |
0.008 |
Tier 2 |
| Isomer of L-Rhamnofuranose |
735.3 |
1.66 |
0.008 |
Tier 2 |
| 6-Deoxy-L-Galactose |
649.6 |
1.66 |
0.008 |
Tier 2 |
| D-Galactosamine |
162.8 |
1.48 |
0.008 |
Tier 2 |
| Sepiapterin |
240.1 |
1.48 |
0.014 |
Tier 2 |
| Isomer of Sepiapterin |
182.5 |
1.46 |
0.028 |
Tier 2 |
| L-Fuculose |
128.6 |
1.43 |
0.001 |
Tier 2 |
| Isomer of Deoxyadenosine |
261.6 |
1.42 |
0.019 |
Tier 2 |
| Deoxyadenosine |
243.3 |
1.40 |
0.035 |
Tier 2 |
| 3-Hydroxy-L-Proline |
211.6 |
1.23 |
0.016 |
Tier 2 |
| N-Acetyl-trans-3-Hydroxy-L-Proline |
602.8 |
0.82 |
0.080 |
Tier 2 |
| Ethanolamine |
398.0 |
0.82 |
0.045 |
Tier 2 |
| 2,3-Dihydroxyindole |
578.9 |
0.81 |
0.044 |
Tier 2 |
| N2'-Acetyl-3'-Hydroxykynurenamine |
425.7 |
0.79 |
0.087 |
Tier 2 |
| Cyanate |
469.9 |
0.79 |
0.014 |
Tier 2 |
| Isomer of Cyanate |
505.6 |
0.77 |
0.008 |
Tier 2 |
| Glycerol |
447.9 |
0.76 |
0.051 |
Tier 2 |
| Alpha-Ribazole |
894.1 |
0.74 |
0.014 |
Tier 2 |
| Isomer of 3-Phenoxybenzyl Alcohol |
538.1 |
0.73 |
0.008 |
Tier 1 |
| Dihydroshikonofuran |
1341.0 |
0.73 |
0.018 |
Tier 2 |
| Glycolaldehyde |
613.0 |
0.71 |
0.018 |
Tier 2 |
| N-Acetyl-2-Carboxy-2,3-Dihydro-5,6-Dihydroxyindole |
404.7 |
0.69 |
0.014 |
Tier 2 |
| Allotetrahydrodeoxycorticosterone 3-O-Glucuronide |
1167.4 |
0.69 |
0.027 |
Tier 2 |
| Phenethyl Alcohol |
653.4 |
0.63 |
0.002 |
Tier 1 |
| Propane-1,3-Diol |
945.6 |
0.56 |
0.001 |
Tier 2 |
| Cortolone |
1011.9 |
0.46 |
0.092 |
Tier 2 |
| Arabitol |
238.7 |
0.42 |
0.001 |
Tier 2 |
| Cortisol 21-O-Sulfate |
875.1 |
0.40 |
0.088 |
Tier 2 |
Table 2: Identified hydroxyl-containing metabolites affected by cannabidiol (CBD) compared to control (CON). Metabolites with a fold change (FC) ≥ 1.2 relative to CON and a false discovery ratio (FDR) ≤ 0.05 considered increased in the CBD compared to CON. Metabolites with a FC ≤ 0.83 and an FDR ≤ 0.05 considered reduced in CBD compared to CON.
1Normalized RT (retention time) shows the corrected retention time of the peak pair with Universal RT Calibrant data.
2Tier 1 indicates positive metabolite identification within the chemical isotope labeling (CIL) metabolite library whereas Tier 2 indicates high confidence putative identification within the linked identity (LI) library.
Volcano plot analysis showed that 32 metabolites were differentially altered (FC ≥ 1.2 or ≤ 0.83, FDR ≤ 0.05) by CBD (Figure 3B; Table 2). Fourteen metabolites were differentially increased (FC ≥ 1.5, FDR ≤ 0.05) by CBD. Eighteen metabolites were differentially reduced (FC ≤ 0.83, FDR ≤ 0.05) by CBD compared with control.
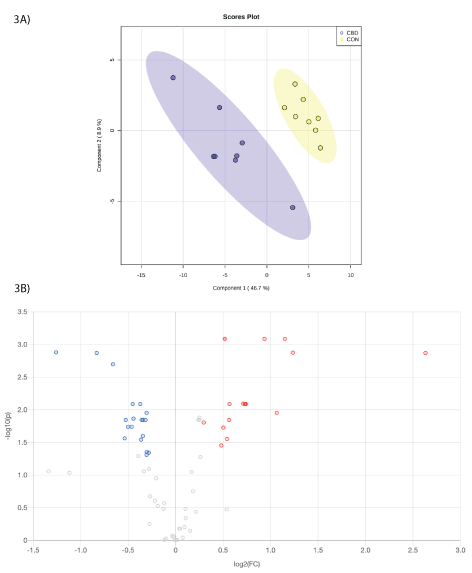
Figure 3: A. Partial least squares discriminant analysis (PLS-DA) scores plot and B. volcano plot showing the differential hydroxyl-containing metabolites. Fold change (FC) ≥ 1.2 (in red) or ≤ 0.83 (in blue) with false discovery ratio (FDR) ≤ 0.05 are differentially increased or reduced by cannabidiol (CBD) relative to control (CON).
Univariate analysis of the 35 hydroxyl-containing metabolites positively and putatively identified that were differentially increased or decreased by CBD revealed that 15 metabolites appear to be highly predictive of the metabolomic changes between CBD and CON (AUROC ≥ 0.90; P< 0.001; Figure 4).
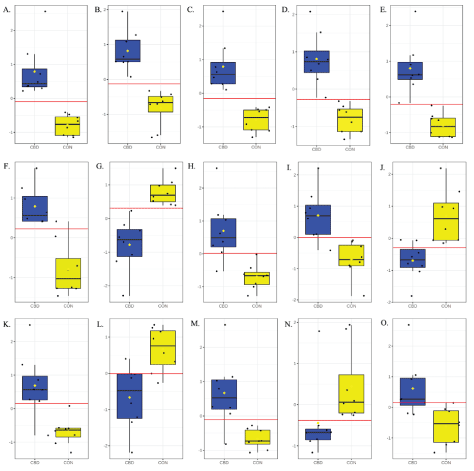
Figure 4: Biomarker analysis of hydroxyl metabolites for CBD (CBD) and control (CON) treatments identified A. L-fuculose (AUROC = 1.00; P < 0.001); B. glyceraldehyde (AUROC = 1.00; P < 0.001); C. L-rhamnofuranose (AUROC = 1.00; P < 0.001); D. L-rhamnono-1,4-lactone (AUROC = 1.00; P < 0.001); E. Arabitol (AUROC = 1.00; P < 0.001); F. D-tagatose (AUROC = 0.98; P < 0.001); G. propane-1,3-diol (AUROC = 0.98; P < 0.001); H. 6-deoxyL-galactose (AUROC = 0.97; P = 0.002); I. D-galactosamine (AUROC = 0.95; P = 0.001); J. phenethyl alcohol (AUROC = 0.94; P = 0.002); K. isomer of L-rhamnofuranose (AUROC = 0.92; P = 0.001); L. 3-phenoxybenzyl alcohol (AUROC = 0.91; P = 0.003); M. 3,4-dihydroxyphenylpropanoate (AUROC = 0.91; P = 0.003); N. cortolone (AUROC = 0.91; P = 0.073); and O. isomer of deoxyadenosine (AUROC = 0.91; P = 0.008) as candidate biomarkers.
Discussion
Lipid metabolism
Increased concentrations of 17α, 20α-dihydroxypregn-4-en-3-one, ethyl malonate, 3-hydroxybutyric acid, an isomer of 3-hydroxybutyric acid, 3-hydroxypropionic acid, an isomer of hydroxypropionic acid, and butyric acid may indicate an alteration of lipid metabolism with CBD supplementation. Primarily known as a steroid hormone elevated in late pregnancy in mammals, 17α, 20α-dihydroxypregn-4-en-3-one is also thought to play a role in progesterone homeostasis in nonpregnant animals [9,10]. Ethyl malonate is a branched fatty acid associated with several fatty acid metabolism disorders in humans, including short-chain acyl-CoA dehydrogenase deficiency and ethylmalonic encephalopathy [11,12], and has also been identified as a potential biomarker for the detection of human breast cancer [13]. 3-hydroxybutyric acid is a ketone body and a typical intermediate in the partial breakdown of branched-chain amino acids like valine in muscles [14]. 3-Hydroxypropionic acid is an intermediate in beta-alanine, propanoate, and uracil metabolism [15,16]. Butyric acid is an intermediate in butanoate metabolism, which is often formed as a result of bacterial fermentation in the gut [17,18]. Many of these changes could also be impacted by changes to the microbiome which has not been previously linked to CBD supplementation.
Additionally, decreased concentrations of propane-1,3- diol, glycerol, ethanolamine, an isomer of 9-oxononanoic acid, 9,10-12,13-diepoxyoctadecanoate, allotetrahydrodeoxycorticosterone 3-O-glucuronide, cortolone, and cortisol 21-O-sulfate may also suggest that CBD altered lipid metabolism. Propane-1,3-diol is a product of glycerol fermentation in bacteria but is not known as a mammalian metabolite [19]. Glycerol is the backbone of glycerides and plays an essential role in lipid metabolism, carbohydrate metabolism, and other metabolic processes [20]. Blood glycerol is also a biomarker for liver disease, hyperglycemia, and type II diabetes [21,22]. Additionally, glycerol is involved in endocannabinoid signaling as a product of the endocannabinoid 2-arachidonylglycerol hydrolysis [23]. Ethanolamine, an amino alcohol, is an intermediate in glycerophospholipid metabolism. It is also involved in retrograde endocannabinoid signaling as a product of arachidonoyl ethanolamide degradation by fatty acid amide hydrolase [24]. Collectively, these results may suggest CBD inhibited arachidonoyl ethanolamide degradation, which supports previous work showing inhibition of cellular uptake and breakdown of arachidonoyl ethanolamide by CBD via inhibition of fatty acid binding proteins [25-27].
9-Oxononanoic acid is a medium-chain fatty acid product of alphalinoleic acid oxidation in plants [28]. Early studies showed elevated lipid peroxides and reduced lipogenesis in rat livers after oral administration of 9-oxononanoic acid [29,30]; more recent work indicates this is a result of arachidonic cascade induction by 9-oxononanoic acid through its activation of phospholipase A2 [31]. If the decrease in 9-oxononanoic acid is a result of CBD supplementation, this may be one potential mechanism contributing to the suspected antiinflammatory effects of CBD. Jasmonic acid is a plant stress hormone synthesized from linolenic acid that serves as a natural pesticide and promotes plant growth [32]. Additionally, jasmonic acid has been suggested to exhibit anti-cancer and anti-inflammatory properties in vitro [33]. 9,10-12,13- Diepoxyoctadecanoate is an intermediate in the conversion of linoleic acid into tetrahydrofurandiols [34].
Allotetrahydrodeoxycorticosterone 3-O-glucuronide is an endogenous neurosteroid that acts as a potent positive allosteric modulator of the GABAA receptor and exhibits sedative, anxiolytic, and anticonvulsant effects [35,36]. Cortolone and cortisol 21-O-sulfate are metabolites in steroid hormone catabolism [37]. Cortolone is often linked to a glucuronide to help in its excretion [38,39], and cortisol 21-O-sulfate may be used as a biomarker for Cushing’s syndrome [37].
Altogether, these results support an effect of CBD on lipid metabolism and may indicate potential mechanisms for its suspected anti-inflammatory effect. However, as the animals on this study were healthy, it is unclear how this would translate to a diseased or aged population in which these metabolomic changes might be more influential, such as animals with Cushing’s disease. Further studies are needed in unhealthy or diseased populations of dogs to determine the potential physiological applications of these potential effects of CBD.
Carbohydrate metabolism
Increased concentrations of lactic acid, acetic acid, D-glycerate, D-tagatose, D-galactosamine, L-rhamnono-1,4-lactone, L-rhamnofuranose, L-fuculose, 6-deoxy-L-galactose, and 5-deoxy-Dglucuronate may indicate that CBD altered carbohydrate metabolism. Lactic acid is generated from pyruvate during anaerobic conditions in the muscle and red blood cells. The Cori cycle, or lactic acid cycle, removes lactate from tissues and transports lactate via blood to the liver where it can be converted back into glucose [40,41]. As both this cycle and lactic acid play vital roles in glucose homeostasis, the increase in plasma lactic acid indicates alteration of glucose metabolism by CBD. Similarly, acetic acid plays a role as an intermediate in several essential metabolic pathways, including glycolysis, pyruvate metabolism, and glyoxylate metabolism via acetyl-CoA. These results support our previous work investigating the impact of CBD supplementation on amine/phenol- and carbonyl-containing metabolites, in which glucose and several other metabolites associated with energy metabolism were altered [6], and highlight the importance of continued work on the potential anti-obesity and antidiabetic effects of CBD.
D-glycerate is an intermediate in several metabolic pathways. Regarding carbohydrate metabolism, D-glycerate is an intermediate in the conversion of other D-sugars and glyoxylate to 2-phosphoglycerate, which can then feed into glycolysis [42,43]. D-tagatose is a monosaccharide often found in fruit or dairy products that are commonly used as an artificial sweetener due to its low glycemic index [44]. It can also be produced as an intermediate in the catabolism of D-sugars like D-galactose before entry into the pentose phosphate pathway [45]. D-galactosamine is an amino sugar derived from galactose that is a constituent of glycoprotein hormones like luteinizing hormone and follicle-stimulating hormone [46,47].
L-rhamnono-1,4-lactone, L-rhamnofuranose, L-fuculose, and 6-deoxy-L-galactose (i.e. fucose) are intermediates in fructose and mannose metabolism [48-50]. Fucose, in particular, is a common N-linked glycan that has been shown to play an important role in mammalian health, with disruption of fucosylated glycan expression implicated in a number of disease mechanisms [48,51].
5-deoxy-D-glucuronate is an intermediate in the catabolism of myoinositol in bacterial species like Bacillus subtilis, a common species found in the gastrointestinal tract of humans and ruminants [52,53]. While not known to be produced in canine metabolic pathways, production of 5-deoxy-D-glucuronate in the gastrointestinal tract by micro flora may have been absorbed into the body. The increase in this metabolite may suggest CBD supplementation affected gastrointestinal micro flora populations.
Additionally, decreased concentrations of arabitol may suggest an impact of CBD on the microbiome. Arabitiol is a sugar alcohol commonly produced by several yeast species [54]. Elevated levels of arabitol in urine have been suggested as a biomarker of fungal infections [55], and elevated plasma arabitol has been linked to other diseases like congenital liver cirrhosis and acute mountain sickness in humans [56,57]. While CBD is believed to be beneficial in an assortment of gastrointestinal conditions [58], little work has been done regarding its influence on microbial populations in the canine gastrointestinal tract, highlighting a potential avenue for future investigation of CBD supplementation.
Amino acid metabolism
Increased concentrations of 2,5-dioxopentanoate, 3-hydroxy L-proline, D-glycerate, isomer of D-glycerate, glycolate, isomer of glycolate, glyoxylate, 2-hydroxy-3-oxopropanoate, glycolaldehyde 3,4-dihydroxymandelic acid, hydroxyisobutyric acid, isovaleric acid, butyric acid, S-5-amino-3-oxohexanoic acid, 3-hydroxypropionic acid, and isomer of hydroxypropionic acid all indicate that CBD altered amino acid metabolism.
2,5-dioxopentanoate is an intermediate in the catabolism of 4-hydroxyproline in bacteria [59]. It can be converted into 2-oxoglutarate, by which it can enter the citric acid cycle through conversion into succinyl-CoA in several prokaryotic organisms, including several species of Pseudomonas, Escherichia coli, and Haloferax volcanii [60-62]. Isovaleric acid is a branched-chain fatty acid intermediate in leucine catabolism [63], and S-5-Amino-3- oxohexanoic acid is an intermediate in lysine degradation [64,65].
3-Hydroxy-L-proline is an intermediate in arginine and proline metabolism [66]. D-glycerate is an essential intermediate in the catabolism of glycine, serine, and threonine in plants. Once formed, D-glycerate can then feed into either glyoxylate metabolism via hydroxypyruvate or be converted into 3-phospho-D-glycerate and fed into glycolysis [67,68]. In humans with a glycerate kinase mutation, D-glycerate levels are elevated to the point of acidemia which, if left untreated, can lead to progressive neurological impairment, hypotonia, seizures, failure to thrive, and metabolic acidosis [69,70].
In plants, glycolate is an intermediate in glyoxylate metabolism, converting hydroxypyruvate into glyoxylate for further metabolism in the glyoxylate cycle [71]. In mammalian systems, glyoxylate is produced either through oxidation of glycolate in peroxisomes or the catabolism of hydroxyproline before being converted into glycine via alanine-glyoxylate aminotransferases present in peroxisomes [72,73]. The glyoxylate cycle, a pathway that converts fatty acids into glucose once believed to be absent in mammalian systems, may be present in the liver [74,75]. Genetic defects in glyoxylate metabolic enzymes have been attributed to metabolic diseases like primary hyperoxalurias and insulin resistance, though this has not been investigated in canine models [73,75]. Additionally, plasma glyoxylate has been indicated as an early marker for type II diabetes development in humans [76,77]. 2-Hydroxy-3-oxopropanoate and glycolaldehyde are also additional intermediates in glyoxylate metabolism [78,79].
Glycolaldehyde is also a precursor for pyridoxine synthesis and is an intermediate in folate synthesis in bacteria [80]. 3,4-Dihydroxymandelic acid is a metabolite of norepinephrine with potent antioxidant activity [81]. Hydroxyisobutyric acid is an intermediate in valine degradation that in humans is used as a biomarker of 3-hydroxyisobutyric aciduria and methylmalonic semialdehyde dehydrogenase deficiency, rare metabolic diseases [82]. Alpha-hydroxyisobutyric acid was shown to be upregulated in a small group of dogs with diabetes compared to healthy controls [83], though it was not identified as a potential CBD biomarker in this study.
Decreased concentrations of 4-oxoproline, L-1-pyrroline-3- hydroxy-5-carboxylate, isomer of 1-pyrroline-2-carboxylate, N-acetyltrans-3-Hydroxy-L-proline, arginine, 2-aminomuconate semialdehyde, isomer of 1-aminocyclopropane-1-carboxylate, L-allothreonine, isomer of aspartate, 2-oxo-4-phenylbutyric acid, 2,3-dihydroxyindole, N2’-acetyl-3’-hydroxykynurenamine, cyanate, isomer of cyanate, and N-acetyl-2-Carboxy-2,3-dihydro-5,6-dihydroxyindole may also point to an alteration of amino acid metabolism by CBD. 4-oxoproline, L-1- pyrroline-3-hydroxy-5-carboxylate, and 1-pyrroline-2-carboxylate are intermediates in arginine and proline metabolism [84-86]. N-Acetyltrans-3-hydroxy-L-proline (i.e. oxaceprol) is a derivative of L-proline that is an established anti-inflammatory drug used in the treatment of osteoarthritis [87]. Combined with the decrease in arginine, these results suggest an impact of CBD on arginine and proline metabolism; however, since the relative concentration of proline was unaffected by treatment, the biological significance is unclear.
2-Aminomuconate semialdehyde, 2,3-dihydroxyindole, and N2’- acetyl-3’-hydroxykynurenamide are intermediates in tryptophan metabolism. 2-Aminomuconate is part of the kynurenine pathway, a metabolic pathway used for NAD biosynthesis [88,89]. Kynurenine pathway metabolites like 2-aminomuconate are thought to help regulate processes like immune cell response, neuronal excitability, and host-microbiome signaling [90]. N2’-Acetyl-3’- Hydroxykynurenamine is an acetylated intermediate in tryptophan metabolism [91]. 2-3-Dihydroxyindole is an intermediate in an indole degradation pathway present in several bacterial species but is not known as a mammalian metabolite [92].
2-Oxo-4-phenylbutyric acid is an intermediate in phenylalanine, tyrosine, and tryptophan metabolism that, in microbial species, is a precursor to homophenylalanine [93]; however, this pathway is not known to be present in mammalian species. N-Acetyl-2-Carboxy2,3-dihydro-5,6-dihydroxyindole (i.e. leucodopachrome) is an intermediate in tyrosine metabolism and in the betalain melanogenesis pathway [94,95]. Cyanate is an intermediate in nitrogen metabolism that can be produced spontaneously from urea. It has been suggested to improve insulin sensitivity and potentially exert antioxidative effects [96].
Vitamin and nucleotide metabolism
Increased concentrations of an isomer of threonate, 2-hydroxy3-oxopropanoate, nicotinate, 3-oxopropanoate, an isomer of 3-oxopropanoate, deoxyadenosine, and an isomer of deoxyadenosine may suggest an alteration of vitamin and nucleotide metabolism by CBD. Threonate is an intermediate in ascorbate metabolism that is thought to play a role in bone mineralization, prevent androgendriven balding, and improve learning and memory when combined with magnesium [97-99]. Nicotinate, or vitamin B3, is required for the formation of coenzymes NAD and NADP. While it can be synthesized from tryptophan in humans and dogs, the process is inefficient and is thus considered an essential nutrient [100,101].
3-Oxopropanoate is an intermediate in uracil and beta-alanine metabolism that can then be converted into malonate and enter fatty acid biosynthetic pathways [102]. Deoxyadenosine is a derivative of adenosine and an intermediate in purine metabolism. In the absence of adenosine deaminase in humans, deoxyadenosine will accumulate in and kill T lymphocytes, leading to the development of adenosine deaminase severe combined immunodeficiency disease [103].
Decreased concentrations of alpha-ribazole also indicate CBD altered vitamin metabolism. alpha-Ribazole is an intermediate in vitamin B12 synthesis in plants and bacterial species that can be used as a marker for vitamin B12 content in foodstuffs [104,105].
Additional metabolites
Increased relative concentrations of 4-coumarate, 3,4-dihydroxyhpenylpropanoate, isovanillic acid, citramalic acid, sepiapterin, and an isomer of sepiapterin may further suggest an alteration of metabolism by CBD supplementation. 4-Coumarate, or 4-hydroxycinnamate, is a hydroxycinnamic acid derivative and an intermediate in several plant metabolic pathways, including phenylpropanoid biosynthesis, isoquinoline alkaloid biosynthesis, tyrosine metabolism, and more [106,107]. 3,4-Dihydroxyphenylpropanoate, or dihydrocaffeic acid, is an intermediate in tyrosine metabolism, specifically the conversion of L-DOPA to rosmarinate, and is suspected to possess antioxidant and anti-inflammatory properties [108,109].
Isovanillic acid is a metabolite of isovanillin with suspected antibacterial properties [110,111]. While not known as a mammalian metabolite, it can be found in plasma after flavonoid consumption [112]. Citramalic acid is most commonly produced by yeast or anaerobic bacteria as an intermediate in C5-branched dibasic acid metabolism [113]. As it is not a mammalian metabolite, citramalic acid is most commonly found as a urine metabolite in cases of yeast or bacterial overgrowth [114]. Sepiapterin is an intermediate in folate biosynthesis that in mammals can be metabolized into tetrahydrobiopterin, which is an essential cofactor in mammals for the metabolism of aromatic amino acids and biosynthesis of neurotransmitters [115,116]. It has been suggested as a treatment for atherosclerosis and tetrahydropterin deficiencies in humans [115-118].
Decreased concentrations of L-glutamate-1-semialdehyde, 1-aminocyclopropane-1-carboxylate, L-allothreonine, an isomer of 3-phenoxybenzyl alcohol, dihydroshikonofuran, and phenethyl alcohol also indicate an effect of CBD on metabolism.L-Glutamate1-semialdehyde, 1-aminocyclopropane-1-carboxylate, and L-allothreonine are plant metabolites. L-Glutamate-1-semialdehyde is produced as an intermediate in porphyrin and chlorophyll synthesis [119]. 1-Aminocyclopropane-1-carboxylate is the direct precursor of the plant hormone ethylene and plays a role in the regulation of plant development [120]. L-Allothreonine is a stereoisomer of threonine and an intermediate in plant glycine, serine, and threonine metabolism [121]. 3-Phenoxybenzyl alcohol is a mammalian metabolite of the insecticide permethrin produced by carboxylesterases [122,123]. Dihydroshikonofuran is a monoterpenoid product of the ubiquinone biosynthetic pathway that also produces shikonin, a plant pigment with anti-inflammatory, antibacterial, and wound-healing properties [124,125]. Phenethyl alcohol is a natural fragrance produced by rose, carnation, and other plants [126,127].
Limitations
While this analysis provides a comprehensive scan of potential metabolic targets in dogs receiving CBD, it was not intended to be all-encompassing. Because of the lack of metabolic profile with CBD, this was intended to be an exploratory first look into the potential for CBD supplementation to alter the canine metabolome. Limitations of this study include the relatively small sample size, lack of baseline measurement, short duration of CBD supplementation, and the use of only a single dose (mg/kg) of CBD. Additionally, as neither the treats nor CBD extract were analyzed, it is possible that the increased metabolites were present as a result of CBD extract supplementation rather than an alteration of metabolism. Even so, identifying changes in the metabolome is essential for directing future targeted investigations into both the physiological relevance of these changes as well as elucidating potential mechanisms leading to these observed effects. It would be beneficial for future work to evaluate metabolomic changes in an unhealthy or diseased population of dogs supplemented with CBD and to investigate potential differences between short- and long-term CBD administration.
Conclusions
This analysis showed an alteration of canine carboxyl and hydroxyl submetabolomes after 3 weeks of 4 mg CBD/kg BW/d supplementation. Altered metabolites may suggest a potential for CBD to influence lipid, carbohydrate, amino acid, vitamin, and nucleotide metabolism as well as possible links with the host mcrobiome. Several metabolites were identified as potential biomarkers for changes in the canine metabolome by CBD that may be used in future work using a targeted metabolomics approach. Additionally, changes in relative concentrations of metabolites like acetic acid, 9-oxononanoic acid, and 3,4-dihydroxymandelic acid may indicate potential pathways by which CBD may exert suspected anti-inflammatory, antioxidant, and anti-obesity effects. Future work would benefit from larger sample sizes, longer supplementation periods, and multiple CBD doses.
Author Contributions
DH, EM, KM, and SK contributed to the conception and design of the study. EM, SK, and DS facilitated data collection. EM and IO performed statistical analysis. EM wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The authors declare that this study received funding from AgTech Scientific, Paris, KY. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the venue for publication.
Acknowledgements
We thank Lincoln Memorial University students K Athey, H Barnhart, L Calvin, K Dubois, J Gauldin, S Swears, M Kight, M Mendoza, J Steen, S Swears, and K Williams for their assistance in caring for dogs and facilitating data collection.





