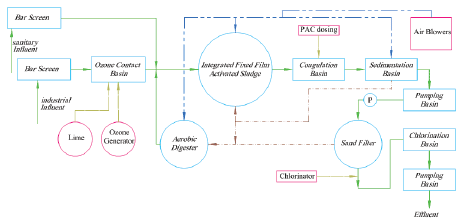
Figure 1: Flow schematic of the Iran Gelatin Capsule WWTP

Ahmad Sabzali1* Nasrollah Amin Gheidari2
1Ph.D of Environmental Health engineering, Isfahan University of medical science, Iran*Corresponding author: Ahmad Sabzali, Ph.D of Environmental Health engineering, Isfahan University of medical science, Iran, Tel: +98 2144012864; E-mail:ahmad13892010@hotmail.com
Gelatin is one of the most consumed colloid protein material in pharmaceutical, medical, food and military industries. Washing of machines and reactors in pharmaceutical hard gelatin capsules production factories produce a high strength colored wastewater with high COD and TSS concentrations, low pH and a high protein content. The main objective of this study was to evaluate the performance of Iran Gelatin Capsule wastewater treatment plant (IGCWWTP) in terms of COD, TSS, ammonium, and phosphorus removal and chemicals and energy consumption.
An intensive analytical program was followed for more than 600 days for monitoring IGCWWTP influent and effluent. This study revealed that average concentrations of COD, TSS, ammonium, and phosphorus removal in the effluent from the effluent plant were 41.8 mg/l, 9.1mg/l, 0.48 mg-N/l, 1.6 mg P-PO4, respectively. The average electricity consumption and potable water usage per influent wastewater flow rate were 0.33 kWh/m3 and 37 L/m3 , respectively. The average lime, PAC, and chlorine consumption per influent wastewater flow rate were 0.2 Kg/m3 , 0.05 Kg/ m3 , 0.0 Kg/m3 , respectively.
Wastewater; Pharmaceutical-capsule production industry; Kaldnes-K3; IFAS
Pharmaceutical wastewater treatment due to high concentration of non-biodegradable compounds is an environmental problem and requires the application of integrated processes such as combination of advanced oxidation processes with biological treatment for treating industrial wastewater [1].
Gelatin is one of the most consumed colloid protein material in pharmaceutical, medical, food and military industries [2].
The first step in capsule production is to form a hard gelatin shell. The shells are produced by machines that dip rows of rounded metal dowels into a molten gelatin solution, and then strip the capsules from the dowels after the capsules have cooled and solidified. Imperfect capsules are remelted and reused, if possible, or sold for glue manufacture [3]. Washing of machines and reactors produce a high strength colored wastewater with high COD and TSS concentrations, low pH and a high protein content.
Iran Gelatin Capsule mfg Co. is the only pharmaceutical hard gelatin capsules production factory in Iran. Its annual capacity is 4.2 billion empty capsules in different sizes and colors.
An underground wastewater treatment plant (WWTP) was established in the factory in 2011 to treat sanitary and industrial wastewater generated in the factory. The WWTP has been designed as chemical/biological processes, which consists of one advanced oxidation reactor, two biofilm reactors, one coagulation reactor, and filtration unit.
The biofilm reactors have been designed as integrated fixed film activated sludge (IFAS) with moving media.
IFAS added the benefits of biofilm reactors to the suspended growth activated sludge process [4,5]. This process is designed to enhance the activated sludge process by providing a greater concentration of biomass to the packing material placed in the mixed liquor.
The combination of suspended and attached growth biomass increases the carbonaceous removal, in terms of the organic loading rate (OLR) and promotes more advanced wastewater treatment by the longer SRT [6]. Furthermore, biofilm treatment processes are stable and resistant to organic and hydraulic shock loadings [7].
Biofilm carrier particles (media) are an important component of the IFAS system. Several types of synthetic packing materials have been used as carriers that may be suspended or fixed in the aeration tank of the activated sludge process [4]. Moreover, the applications of inexpensive and available bio-carriers such as pumice stone, porous glass beads, FLOCOR-RMP®, biodegradable meal box, liquorice (Glycyrrhiza glabra), giant reed (Arundo donax), cotton, and cigarette filter rods are the key factors for the adoption and application of biofilm reactors in developing countries [8-12].
This study was conducted to evaluate the performance of the WWTP in Iran Gelatin Capsule factory in terms of COD, TSS, ammonium, and phosphorus removal and chemicals and energy consumption.
The existing WWTP is a chemical/biological treatment plant with an approximate design capacity of 80 m3 /d (Figure 1). Wastewater was entered into the WWTP through gravity in two separate sewers. Industrial wastewater was conducted by a polyethylene pipe into bar screen channel. After screening, the wastewater was entered into an advanced oxidation basin of volume 38 m3 for breaking down long chain of pollutant to smaller chains. After that, the pretreated industrial wastewater was combined with screened sanitary wastewater and the combined influent was entered into two aeration basins of volume 101 m3 . A constant flow rate of lime solution was directly pumped into aeration basin for adjusting pH. Biological treatment of the combined wastewater was done by integrated fixed film activated sludge (IFAS) process in two aeration basin filled by 7 m3 of Kaldnes-K3 plastic media (Table 1). For better sedimentation of detached bio-flock, a coagulation basin of volume 0.125 m3 was constructed after second aeration basin. Poly aluminum chloride with the concentration of 2-8 mg/l was injected into biological treated wastewater. The concentrated sludge in the bottom of sedimentation basin was returned into the first aeration basin by two air-lift pump. One blower with air flow rate of 7.6 m3 /min was used for mixing and oxygen supply of the aeration basins.

Figure 1: Flow schematic of the Iran Gelatin Capsule WWTP

Table 1: Characteristics of the bio-carriers
a) Quantity in the reactor, b) Bulk volumetric filling, c) Total Surface, d) Protected surface
A pressure sand filter was used for final polishing of the treated effluent. After disinfection with chlorine, the treated effluent was used to irrigate of tree’s factory.
The performance of WWTP was evaluated by daily sampling of the influent and effluent at different steps. On every workday, the temperature, dissolved oxygen (YSI 55 DO meter) and pH (SCHOTT pH meter model CG-824) were measured in each reactor.
The soluble chemical oxygen demand (SCOD) was measured immediately after the filtration of the samples by the closed reflux colorimetric method. The determination of ammonium (NH4 -N), and phosphorus (PO4-P) were completed by using reagent kits (model 8075, 10049 and 8114 Hack Company, respectively). The MLSS and MLVSS were analyzed according to standard analytical procedures [13].
The fixed biomass concentration in the IFAS reactors was obtained by the difference in the dry weight between the bio-carriers and the control bio-carriers after drying samples at 70 to 80°C for 1 week [14]. The IFAS process kinetics with regard to COD removal was calculated using the Stover-Kincannon and second order kinetic models. The statistic twotailed Student t-test was used for performance comparison between two reactors. The HRT was calculated using the following Equation (1) [15]:
$$HRT = {{V\left( {{m^3}} \right)} \over {Q\left( {{\raise0.5ex\hbox{$\scriptstyle {{m^3}}$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle d$}}} \right)}} \times 24.........\left( 1 \right)$$
The chemical materials and solution that were used in WWTP were lime, ploy aluminum chloride (PAC), chlorine powder, and potable water.
The potable water was used for preparing chemical coagulant, chlorine solution and cleaning surface of WWTP. The electricity consumption and potable water usage per influent wastewater flow rate ranged from 0.1 to 1.8 kWh/m3 (average: 0.33 kWh/m3 ) and 0.0 to 214 L/m3 (average: 37 L/ m3 ), respectively.
Influent pH value was changed from 4 to 11. This fluctuating pH has been reported by Wurster and coworkers [16]. The influent pH value was increased up to 9.0 after any organic shock load. The effluent pH of ozone reactor was adjusted to 6.5 by adding lime solution with average quantity of 109 g/m3 . The Effluent TSS concentration was 5.2 to 22.6 mg/l (average: 9.1 mg/l).
The average lime, PAC, and chlorine consumption per influent wastewater flow rate were 0.2 Kg/m3 , 0.05 Kg/m3 , 0.01 Kg/m3 , respectively.
COD removal: Influent COD concentration was changed from 1706 to 2752 mg/l (average: 2180 mg/l; STD: 156 mg/l). The effluent concentrations of COD in ozone and IFAS reactors were monitored during the operation. Figure 2 shows the variation of effluent COD concentration of IFAS reactor against operation time.
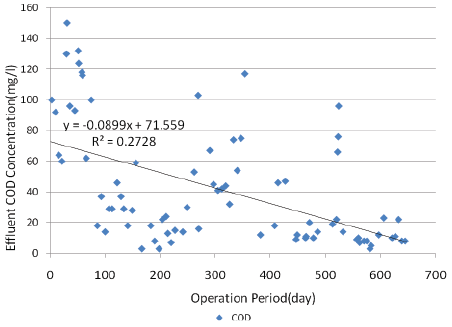
Figure 2: Variation of effluent COD concentration of IFAS reactor against operation time
The Effluent COD concentration was 1484 to 2395 mg/l (average: 2009 mg/l) for ozone reactor and 3 to 150 mg/l (average: 41.8 mg/l) for IFAS reactor.
The OLR into aeration basin and on moving media surface was changed from 0.1 to 2.4 kg COD/m3 .d (average: 0.7 kg COD/m3 .d) and 2.9 to 70g COD/m2 .d (average: 19.7 g COD/m2 .d), respectively.
The HRT was changed from 7.8 h to 7.6 d (average: 28 h) for ozone reactor and 20.7 h to 20.2 d (average: 75 h) for IFAS reactor because of daily influent variations.
The MLSS concentration was 5420 to 7805 mg/l (average: 6410 mg/l) under normal operating conditions. The SRT value was 38-49 days (average: 41.4 days) under normal operating conditions.
Nitrogen and phosphorous removal: The effluent concentration of NH4 -N was monitored during the last 130 days of operation period (Figure 3). The average removal rates of NH4 -N was 98.4% (max=99.6%, min=72.3%, std. dev. of 4.4%). These values are related to an average inflow NH4 -N concentration of 24.8 mg NH4 /l-N (max= 32.1 mg/l, min=17.3 mg/l, std. dev. of 5.9 mg/l).
The Effluent NH4 -N concentration was 0.13 to 4.19 mg-N/l (average: 0.48 mg-N/l, std. dev. of 0.7 mg-N/l) for ozone reactor and 3 to 150 mg/l (average: 41.8 mg/l) for IFAS reactor (Figure 3).
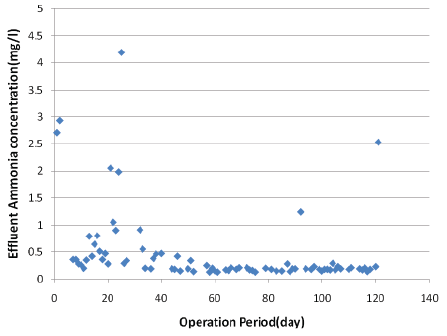
Figure 3: Effluent NH4 -N concentration variations against operation time
The WWTP achieved average phosphorous removals of 86.6% (max=97.1%, min=62.7%, std. dev. of 6.3%). These values are related to an average inflow P-PO4 concentration of 11.7 mg P-PO4 (max=21.3 mg/l, min=7.9 mg/l, std. dev. of 4.6 mg/l).
Kinetic models: The Stover-Kincannon model (Equation 2) is the most widely accepted method in modeling a variety of different types of biofilm reactors [17]. The second order kinetic model (Equation 3) was also utilized for data obtained from the experimental studies [18].
$${V \over {Q\left( {{S_i} - {S_e}} \right)}}\, = {{{K_B}} \over {{U_{\max }}}}\left( {{V \over {Q{S_i}}}} \right) + {1 \over {{U_{\max }}}}.........\left( 2 \right)$$
$${{HRT \times {S_i}} \over {{S_i} - {S_e}}}\, = b \times HRT + a.........\left( 3 \right)$$
Where V, Q, Si, Se, KB, Umax, HRT, a and b are reactor volume (l), inflow rate (l/d), influent substrate (mg/l), effluent substrate (mg/l), saturation value constant, maximum specific substrate utilization rate, Hydraulic retention time (day) and kinetic constants, respectively.
Stover-Kincannon model: In the Stover-Kincannon model, [V/(Q(SiSe))] was plotted versus V/(QSi). In Figure 4, linear regression was used to calculate the slope (KB/Umax) and the intercept point of the straight line (1/Umax) [8,20]. According to the straight line in the Figure 4, KB and Umax are 74.35 g/L.d and 74.62 g/L.d, respectively.
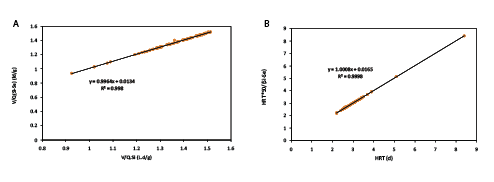
Figure 4: Stover-Kincannon (A) and second order kinetic (B) model plots for COD removal in IFAS reactor with Kaldnes-K3
Second Order Kinetic Model: The ability of the second order kinetic model was evaluated for the IFAS reactors. The values for a and b were calculated from the intercept and slope of the straight line on the graph (Figure 3). The relationship between Se, S0 and HRT is represented in a second order kinetic form for the IFAS reactor with kaldnes media in Equation 4.
$${S_{\bmod el}}\, = {S_o}\left( {1 - {{HRT} \over {0.0165 \times HRT + 1.0008}}} \right)\,.........\left( 4 \right)$$
Where, S0 is the substrate concentration (mg/l).
The average efficiency of the COD removal was 8.6% (Std. Dev. of 3.8%) and 97.9% (Std. Dev. of 1.8%) for ozone reactor and IFAS reactor, respectively. The maximum COD removal efficiencies (99.8%) were observed at an OLR of 0.68 Kg COD/m3 .d for IFAS reactor. The COD removal efficiency for the highest OLR (2.4 Kg COD/m3 .d) was approximately 10% and 93.2% for ozone reactor and IFAS reactor, respectively. However, there is little change in the removal efficiency with an increasing influent COD concentration.
The high COD removal efficiency in IFAS reactor is dependent on the higher surface area and the entrapment ability of moving media [12]. The moving bio-carrier with a higher colonized specific surface could increase the volumetric loading removal efficiency and cause higher treatment capacity [20].
The effluent generated in the gelatin industries is rich in protein, presenting high values of ammonia and general processes such as conventional aerated ponds couldn’t obtain a good removal of ammonia [21]. As shown in Figure 3 the integration of advanced oxidation and IFAS reactor can be a good option for removing ammonia from gelatin-rich wastewater.
In Stover-Kincannon Model the linear regressions with high R2 for the IFAS reactor showed that the COD removal efficiency was a function of organic loading rate.
The Second Order Kinetic Model showed that the COD removal efficiencies increased with the increase in the HRT and the decrease of the influent COD concentration.
The overall performance of IGCWWTP was very good as it treat the high strength wastewater to meet the effluent standards of COD (200 mg/l) and ammonia (1 mg-N/l). On the other hand, this investigation demonstrated that the combination of advanced oxidation process with IFAS bioreactors can endure strong organic and hydraulic loading impact, even though the OLR was sharply increased 2 times; the system could recover the normal treat efficiency in 2days.
This research was conducted with funding from the Iran Gelatin Capsule mfg Co.
Download Provisional PDF Here
Article Type: Case Report
Citation: Sabzali A, Gheidari NA (2016) A FullScale Chemical/Biological Treatment System Application for the Wastewater Treatment of a Pharmaceutical-Capsule Production Industry-A Case Study. Int J Water Wastewater Treat 2(3): doi http://dx.doi.org/10.16966/2381-5299.122
Copyright: © 2016 Sabzali A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access