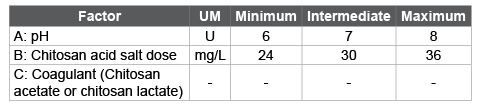
Table 1: Selected conditions for the experimental design

Mario A García1* Isabel Montelongo2 Alejandro Rivero2 Nilia de la Paz3 Mirna Fernández1 Margarita Núñez de Villavicencio2
1Pharmacy and Food Institute, University of Havana, Havana, Cuba*Corresponding author: Mario A García, Pharmacy and Food Institute, University of Havana, St. 222 No. 2317, ZC 13600, Havana, Cuba, Tel: +53 7 2716389; Fax: +53 7 2603894; E-mail: marioifal@gmail.com
The effect of chitosan acetate and chitosan lactate prepared from chitosan obtained by N-deacetylation of chitin from common lobster (Panulirus argus), as coagulants/flocculants for depurating wastewater of fish processing industry was evaluated. Experiments were carried out by Jar tests varying chitosan acid salts concentration and pH. The results were evaluated in terms of reduction of total suspended solids (TSS) and chemical oxygen demand (COD) of the wastewater. Response surface methodology was applied to obtain quadratic and second-degree response surface model equations. With both chitosan acid salts, it was obtained a decrease in TSS and COD between 70-90% and 26-30%, respectively was observed. Optimal values for coagulation-flocculation process, found it by numerical optimization, were: a) for chitosan lactate, pH=6.0 and salt dose of 29.50 mg/L, with values for COD of 30.4% and TSS of 89.5%; and b) for chitosan acetate, pH=7.13 and salt dose of 28.29 mg/L, with values for COD of 23.9% and TSS of 84.0%. Chitosan lactate was more effective as coagulant than chitosan acetate for decreasing the COD and TSS, due to that lactate ion exerts a higher electrostatic force over the electric charges of the particles in suspension, favoring the flocculation process.
Chitosan acetate; Chitosan lactate; Wastewater treatment; Coagulation; Flocculation
Water is considered today a scarce resource; hence its cost is increasingly high. About the 40% of the world population does not have basic sanitation and one in five people on the planet does not have access to clean water. Contrary to what many may think, the water is not an inexhaustible resource that renews itself as a natural and infinite cycle. The problems related to water supply are considered as one of the most serious threats to humanity [1,2].
One of the major challenges currently facing the modern industry is to make compatible with debugging production of waste, emissions and discharges that it generates. Proper treatment of wastewater and industrial wastes and their reuse for multiple uses contributes to sustainable water consumption and environmental regeneration of ecosystems, without forgetting that the water of different quality is a critical raw material for many industries [3].
Within the industrial sector, in the facilities used for processing/ transformation/benefit of fisheries resources, appreciable amounts of liquid and solid wastes from the production process are generated daily. These wastes should be treated by suitable purification processes, some more expensive than others, but all needed to minimize the environmental impact of these waters in the ecosystems [4].
Among the wastes generated in this industrial sector and underutilized in Cuba, until to date, are the remains of exoskeletons, heads and other parts usually inedible from processed marine resources, which are generally drawn and arranged in conjunction with liquid discharges during cleaning processes of production rooms or join discarded resources for breaching the quality standards [4]. Among these, it can be found the chitin, mainly present in the exoskeleton of crustaceans and from whose deacetylation one can be obtained chitosan, a biopolymer with excellent coagulation properties and a wide range of applications [5-9].
Moreover, it has been shown that the coagulation by cationic polymers of high molecular weight that form flocs and tend to settle, as the case of chitosan, provides good results in clarification of industrial wastewater [10,11].
The effectiveness of chitosan as coagulant-flocculant has been tested in a variety of agro food-industry wastewaters [12] such as poultry, meat [13], dairy [13,14], biogases [15] and egg [16] as well as various natural waters with turbidity [17] to give removals between 70 and 90% for total suspended solids content (TSS) and between 60 and 70% for chemical oxygen demand (COD), as fundamental forms used to measure pollution in those cases. Moreover, chitosan and extracted pandan leaves were used as flocculants in the treatment of textile wastewater [18].
Recently, with the development of the nanotechnology, it was evaluated the use of nanofiltration membranes from sulfated chitosan composite [19], chitosan/polyethersulfone composite [20] and chitosan/cellulose acetate composite [21] for wastewater treatment. Moreover, it had informed about the wastewater treatment with chitosan nano-particles [22], immobilized chitosan bio-membrane [23] and chitosan immobilized on silica surface [24].
Although chitosan has been assessed as a coagulant-flocculant in the wastewater treatment, its use is hindered due to its solubility in organic acids, that´s why, in the present work it is proposed the use of chitosan acid salts due to their water solubility.
In this context, and considering that in the specialized literature there is no reports about the use of chitosan acid salts in the wastewater processing, the present work aimed the use of chitosan acid salts prepared from chitosan obtained by N-deacetylation of chitin from common lobster (Panulirus argus) in the removal of TSS and COD in fishery wastewater by coagulation-flocculation process.
Sixty liters of wastewater samples were collected in 20 L-capacity tanks at different areas of the residual treatment facilities of the Industrial Fishing Company La Coloma (EPICOL, Pinar del Río, Cuba). The wastewater samples were stored at 4.0 ± 2°C for a period of 48 h after since they were collected. The wastewater of the three tanks was mixed in a tank of 100 L of capacity and kept remained at 4.0 ± 2°C until its physicochemical characterization.
Chlorides, pH, conductivity, biochemical oxygen demand, chemical oxygen demand (COD), total nitrogen, total phosphorus, total solids, fixed total solids, total volatile solids, total suspended solids (TSS), total dissolved solids, settleable solids and fat were determined for to a representative sample of mixed wastewater according to techniques described by [25]. These determinations were also used as standards of comparison to analyze the efficiency of experimental procedures performed.
CIt was used chitosan acetate and chitosan lactate (30% m/m of chitosan) with 80 and 100% of moisture humidity, respectively, produced at Drugs Research and Development Center (Havana, Cuba) from chitosan from chitin of common lobster (P. argus) of molecular weight of 275 kDa and degree of deacetylation of 75% [26] were used.
In the present work, it was used low doses of chitosan acid salts (Table 1) compared respect to traditional coagulants were used, in order to destabilize colloids and to settle suspended particles. On the other hand, the application of high doses of chitosan re-stabilized colloids [10].
The experimental design matrix (Table 2) and processing the data from each run were carried out by Design Expert 8.0.6 software (Stad-ease Inc., 2011) Numerical optimization method was used through an IV Optimal response surface for the best variant of coagulation-flocculation based on the removing of TSS and COD, generating a mathematical model that describes the changes in the TSS and COD, adjusted as follows:
$$\Delta y = \;{a_o}\, + {a_t}A\, + {a_2}B + {a_3}C + {a_4}AB + {a_5}BC + {a_6}AC + {a_7}{A^2} + {a_8}{B^2} + {a_9}{B^2} + {a_9}{C^2}\left[ {Equation\,1} \right]$$
Where Δy, response variable (COD and TSS); a0 -a9 , coefficients obtained by the model matrix resolution; A, pH value; B, concentration of chitosan acid salt; and C, type of chitosan acid salt (chitosan acetate or chitosan lactate).
The process of coagulation-flocculation of the wastewater was conducted at laboratory scale by the classical methodology of jars test by using a laboratory Floculator with six units of simultaneous treatment. Each unit has a standard paddle stirrer with a speed controller for fast or slow mixing stages of coagulation and flocculation. It also has a timekeeper and an illuminated screen in the back part for observing the appearance of the samples.
For each experimental run, the pH of 1 L of wastewater was adjusted according to the experimental design by adding sulfuric acid (H2 SO4 , 1 N) or sodium hydroxide (NaOH, 6 N). After, the wastewater was introduced into a 1 L-capacity glass vessel. The wastewater was stirred at 200 rpm and the proper amount of chitosan acid salt was added simultaneously near to the stirrer on the vessels of each block.
Although the optimal pH range for removing the colloids depends on the nature of water; according to the literature [27-29], chitosan works effectively, as coagulant, to pH values higher than 5.25. That´s why, in this work, different pH values were tested (Table 1) in the coagulationflocculation process.

Table 1: Selected conditions for the experimental design
The mixture was stirred during 3 min at 200 rpm followed by 30 min at 30 rpm for favoring the union among the flocs, since too intense mixing break the flocs already formed. After, the agitation was stopped and the mixture was allowed to settle during 30 min [18]. Finally, it was taken 50 mL of the supernatant was taken with a volumetric pipette at the same depth in all vessels and content of TSS and COD were determined according to the standards for examining water and wastewater [25]. All runs were carried out by controlling the room temperature at 25°C.
Table 3 informs the physicochemical characterization of wastewater from fish processing industry. The values of these parameters are within the minimum and maximum values reported by Rivera and García [30], except the BOD5 , which reached a lower value. Generally, the residual has a neutral pH and low levels of organic matter expressed as COD and BOD5 .
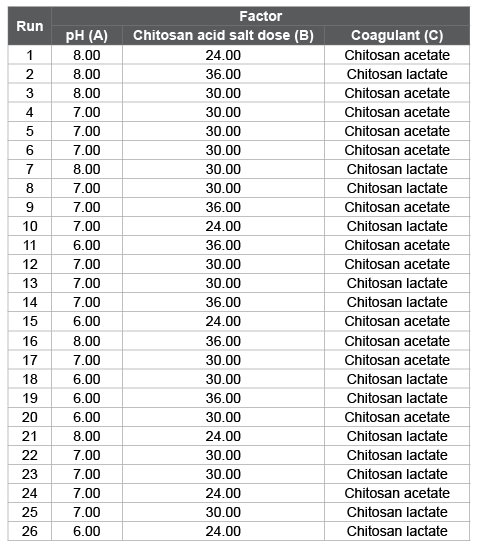
Table 2: Matrix of experimental design
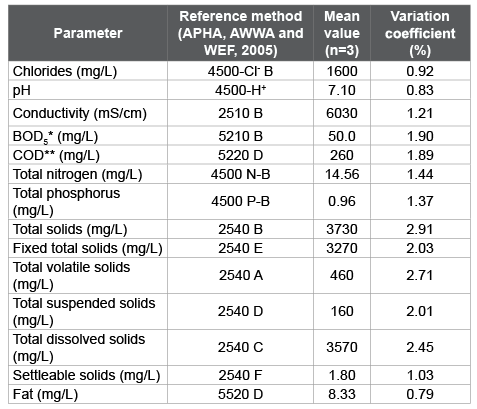
Table 3: Physicochemical characterization of wastewater from fish
processing industry
*BOD5
: biochemical oxygen demand.
**COD: chemical oxygen demand.
The 95.7% of the total solids of the mixed residual are dissolved and in colloidal state while the 4.3% are suspended solids. Also, of the total solids, the 87.7% corresponds to fixed solids, which constitutes the inorganic or mineral portion solids and the remaining 12.3% represents the organic fraction of the solids or solids portion that volatilizes at 550°C.
Table 4 shows the results for the response variables COD and TSS, as well as the values of removal percentages for COD and TSS with respect to the initial values of the residual characterization. The lower values of COD and TSS and consequently the higher removal percentages were obtained for the runs 18 and 19 in the case of the use of chitosan lactate and runs 9 and 15 for chitosan acetate.
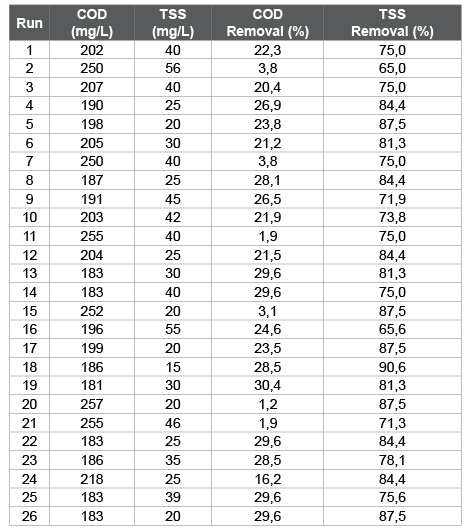
Table 4: Response variables and COD and TSS removal percentage
COD: chemical oxygen demand; TSS: total suspended solids.
Chitosan lactate was more effective as coagulant than chitosan acetate with respect to the TSS and COD removal (Figure 1). For the use of chitosan lactate, the greatest COD apparent removal (30.4%) was obtained at pH=6 and with a coagulant dose of 36 mg/L, while for the TSS removal (90.6%) corresponded with a pH=6 and a coagulant dose of 30 mg/L, although these values may not necessarily be optimal, since they must be obtained through statistical analysis.

Figure 1: Depuration of wastewater from fish processing industry by coagulation-flocculation with (a) chitosan acetate (A) and (b) chitosan lactate (L). To the left, during flocs formation and to the right, after sedimentation. Numbers from 8 to 13 indicate the run according with the matrix of experimental design presented in Table 2.
A possible explanation for the greater effectiveness of chitosan lactate as coagulant may be related to the values of dissociation constant (Ka) of lactic and acetic acids, which are reported as 1.38 × 10-4 and 1.75 × 10-5, respectively at 25°C. Lactate ion has a dipole bond because of its hydroxyl group, which gives a higher electric charge density and thus, has a greater Ka value than acetate ion. Lactate ion attracts water molecules with higher ionic strength, which results in larger contact areas with the colloidal and suspended particles present in wastewater [31], and has a greater electrostatic force on electric charges of these particles, decreasing the energy barrier that prevents them from agglomerating, thus promoting the formation of flocs [32].
The TSS removal (90.6%) was similar to those reported (70-90%) by No and Meyers [13] and Divakaran and Pillai [17] in the treatment of wastewater from other food industries (dairy, poultry and meat), while the COD removal (30.4%) was much lower than reported range (60-70%). The low COD removal percentage can be related with the fact that almost the 96% of the total solids are dissolved in the residual water.
Table 5 shows the significance of analysis of variance of the regression and estimate coefficients for COD and TSS as response variables. It is observed that the obtained model was significant (p ≤ 0.05), showing that there is a significant relationship between COD and TSS with the independent variables of the model. The R2 indicated that the adjusted model explains the 96.3% of the variability in the COD and TSS. The test of lack of fit was not significant (p>0.05). The residue analysis did not show atypical observations and standardized residuals follow a normal distribution with mean zero and standard deviation equal to one.
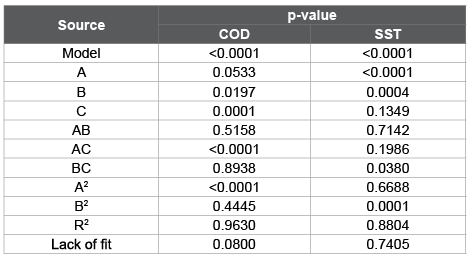
Table 5: Analysis of variance for the COD and TSS of wastewater from fish processing industry
The analysis of the model for COD showed that dose and coagulant type (B, C) as well as the pH-coagulant type interaction (AC) and the quadratic term for the pH (A2) were significant factors (p ≤ 0.05). The pH-coagulant dose (AB), coagulant dose-coagulant type (BC) interactions and the quadratic term for the dose (B2) were not significant (p>0.05). The model equation is:
COD= 193.03 + 3.83A - 4.75B + 6.27C - 1.50AB - 30.33AC - 0.25BC + 28.38A2 + 2.13B2 [Equation 2]
The analysis of the coefficients indicates that the pH-coagulant type interaction has the greatest influence on COD, followed by the quadratic term of pH. According to the sign of the coefficients of the equation, the COD decreases with the increasing of coagulant dose and decreasing of pH, although this occurs only when the chitosan lactate is used as coagulant. In the case of the use of chitosan acetate, the COD decreases with the increasing of coagulant dose and pH, confirming the greater influence of the pH-coagulant type interaction (Figure 2).
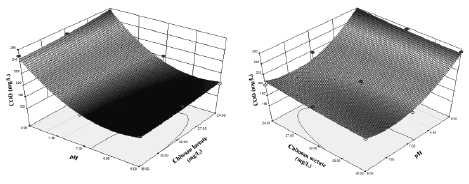
Figure 2: Influence of pH and coagulant dose on COD with a) chitosan lactate, b) chitosan acetate.
The analysis of the model for TSS indicated that pH (A), coagulant dose (B) as well as the dose-coagulant type interaction (BC) and the quadratic term for the dose (B2) were significant (p ≤ 0.05). The pH-coagulant dose (AB) and dose-coagulant type (BC) interactions and the coagulant type (C) were not significant (p>0.05). The model equation is:
TSS= 27.53 + 11.00A + 6.08B - 1.46C - 0.63AB - 1.83AC + 0.88A2 + 3.08BC + 10.13B2 [Equation 3]
From the analysis of the coefficients it is inferred that the pH has the greatest influence on the TSS, followed by the quadratic term of coagulant dose. According to the sign of the coefficients of the equation, the TSS decreases when the pH decreases. The influence of the quadratic term of the coagulant dose was more marked when chitosan lactate was used (Figure 3), decreasing the TSS with the increasing of coagulant dose until a minimum and then increases (parabolic pattern).
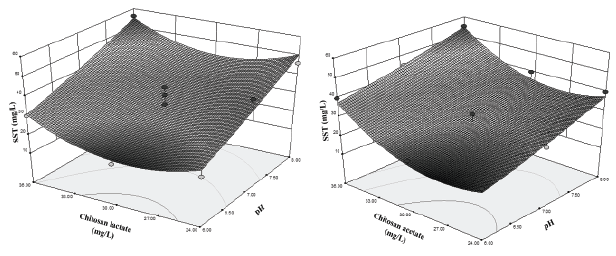
Figure 3: Influence of pH and coagulant dose on TSS with a) chitosan lactate, b) chitosan acetate.
The removal efficiencies increased linearly with increasing pH from 4 to 6 [15]. Increasing the pH up to 6 increases the solubility of chitosan, this increases the protonation of the chitosan surface and enhances the removal efficiencies of turbidity, biochemical oxygen demand (BOD) and COD [33]. In acidic pH, chitosan forms –NH3+ groups that attract the negatively charged organic matter in the wastewater [34], which increased the reduction percentage of turbidity, BOD and COD. The positive charges on the chitosan surface will significantly decrease as solution pH increased [18].
The removal efficiency can be improved with increasing chitosan dose [18,15,16], which is related with the increase of available exchangeable reaction sites [35] for the coagulation process, which considerably reduces the organic matter in the wastewater [36,37].
Chitosan has a high charge density compared with other flocculants such as polyaluminium chloride and polyacrylamide [38]. For that reason, chitosan requires less doses to destabilize the particles [18]. The overdosing of chitosan involves an excess of polymer that is adsorbed on the colloidal surfaces and producing restabilized colloids. Thus there were no sites available on the particle surfaces for the formation of inter particle unions.
The results of the numerical optimization for the coagulationflocculation process that minimizes simultaneously the values of TSS and COD (Table 6), corroborate the greater effectiveness of chitosan lactate as coagulant for fishing wastewater compared to chitosan acetate. In general way, both chitosan acetate and chitosan lactate can be used as coagulant for the treatment of fishing wastewater with removals of COD from 24 to 30% and TSS between 84 and 89% for doses between 28 and 29 mg/L, although the pH shows a significant influence on the process effectiveness in relation with the coagulant type (optimal pH values of 6.0 and 7.13 for chitosan lactate and chitosan acetate, respectively).
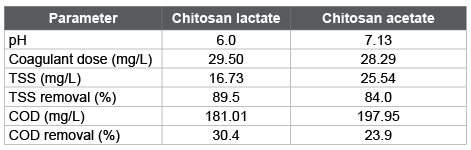
Table 6: Numerical optimization for the coagulation-flocculation process by chitosan acid salts
Chitosan acetate and chitosan lactate may be used as coagulants for decreasing the TSS and COD between 70-90% and 26-30%, respectively, although chitosan lactate was more effective than chitosan acetate, due to that lactate ion exerts a higher electrostatic force over the electric charges of the particles in suspension, favoring the flocs formation. Optimal values for coagulation-flocculation process, found it by numerical optimization, were: a) for chitosan lactate, pH=6.0 and salt dose of 29.50 mg/L, with values for COD removal of 30.4% and TSS removal of 89.5%; and b) for chitosan acetate, pH=7.13 and salt dose of 28.29 mg/L, with values for COD removal of 23.9% and TSS removal of 84.0%.
Download Provisional PDF Here
Article Type: Research Article
Citation: García MA, Montelongo I, Rivero A, de la Paz N, Fernández M, et al. (2016) Treatment of Wastewater from Fish Processing Industry using Chitosan Acid Salts. Int Water Wastewater Treat 2(2): doi http://dx.doi.org/10.16966/2381-5299.121
Copyright: © 2016 García MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access