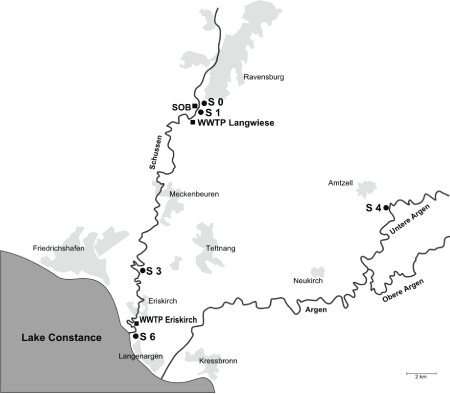
Figure 1: Field sampling sites at Schussen River (up- and downstream of the WWTP Langwiese) and at the reference site at Argen River


Paul Thellmann1* Katharina Greiner-Perth1 Stefanie Jacob1 Marina Knoll1 Manuela Schäfer1 Michael Stängle1 Michael Ziegler1 Marco Scheurer2 Heinz-R.Köhler1 Rita Triebskorn1,3
1 Animal Physiological Ecology, University of Tübingen, Auf der Morgenstelle 5, D-72076 Tübingen, Germany*Corresponding author: Paul Thellmann, Animal Physiological Ecology, Institute for Evolution and Ecology, University of Tübingen, Auf der Morgenstelle 5, Tübingen 72076, Germany, Tel: +4970712978818; E-mail: paul.thellmann@gmx.de
Advanced waste water treatment technologies based on e.g. an additional powdered activated carbon stage are in the focus of today’s science and politics. Despite the abundance of detailed information on the efficiency of these new technologies in the literature, little is known about their effects on the connected surface waters. The present study focuses on a large waste water treatment plant in Ravensburg (Southern Germany), which has been equipped with an additional cleaning stage (powdered activated carbon in late autumn 2013. Within the scope of a pre-post study, effluent samples of this WWTP as well as sediment and surface water samples from the connected River Schussen were investigated by chemical analysis and the fish embryo test with the zebrafish (Danio rerio) over a period of 2 years prior and after the WWTP upgrade. Our results clearly show the additional purification step based powdered activated carbon to result not only in a considerable reduction of micropollutants in the wastewater treatment plant effluent and surface water, but also to improve sediment and surface water quality in respect to a significant reduction of embryotoxic effects. Our study thus revealed the ecological and toxicological relevance of the PAC adsorption technology in wastewater treatment.
Waste water treatment; Tertiary treatment; Powdered activated carbon; Fish embryo test; Sediment; Surface water
Although waste water treatment technologies have been continuously improved over the past 40 years, a large proportion of anthropogenic substances that are present in European surface waters are still released via waste water treatment plants (WWTPs) [1]. The reason for this fact is rooted in the incomplete elimination of various substances during conventional waste water treatment processes [2-4]. As a consequence, anthropogenic substances like personal care products, pharmaceuticals, industrial agents or their transformation products were and are still continuously discharged into the aquatic environment [5,6].
For example, the non-steroidal anti-inflammatory drug (NSAID) diclofenac is a worldwide used pharmaceutical which is often detected in WWTP effluents and in many European surface waters [6,7]. Studies of Schwaiger et al. [8], Triebskorn et al. [9] and Birzle [10] have demonstrated that even low and environmentally relevant concentrations of diclofenac can lead to adverse effects in exposed fish. Endocrine disrupting chemicals (EDCs), like 17α-ethinyl estradiol (EE2) which is a frequent constituent of contraceptives can also be found in many surface waters and WWTP effluents [11,12]. Several studies have demonstrated that already low concentrations (ng/L range) of EE2 are able to adversely affect fish endocrinologically [13-15]. Since many years, environmental pollution with trace substances and waste water treatment with different cleaning technologies have been in the focus of today’s scientists and politicians. This rising interest has various reasons. Probably the most important one is that water resources will play a limiting factor for many regions in the future, especially in the case when the production of raw and drinking water originates from waste water treatment [16]. Particularly in the case of densely populated catchment areas with intense industrial or agricultural land use, the degree of surface waters pollution becomes a crucial factor. In order to improve waste water treatment processes and to meet the requirements of the European Water Framework directive (WFD) additional wastewater treatment technologies based on ozonation or powdered activated-carbon (PAC) were and are currently under research in many studies [17-20]. The advantages, the efficiency, the necessity, and the appropriate application of the available technologies for the reduction of micropollutants and pathogens are a late-breaking topic in sanitary environmental engineering and a matter of intense scientific discussion [21-24]. Whether, and how fast this additional wastewater treatment technologies result in an improvement of ecosystem health, however, is far from being understood.
The present work is part of the joint research program “SchussenAktivplus”. It aims at investigating and assessing differently sized WWTPs in Southern Germany that have been equipped with additional wastewater treatment technologies such as ozonation, powdered activated-carbon (PAC) or granulated activated-carbon (GAC) filtration. The present study focuses on a large WWTP (WWTP Langwiese near Ravensburg, Southern Germany) and the effects of its effluent on the receiving stream, the Schussen River, prior and after the upgrade of the WWTP with an activated carbon stage. The Schussen River is the largest German tributary to Lake Constance, the largest reservoir of drinking water in Germany. The catchment area of the Schussen represents a densely populated and intensely used area which experiences a lot of anthropogenic influences due to the discharge of municipal waste waters. In total 19 WWTPs and 216 storm water overflow basins (SOBs) release their discharges into this river. The WWTP Langwiese represents a large scale plant with a cleaning capacity of 170,000 population equivalents. In late autumn 2013 this WWTP has been upgraded with an additional adsorptive PAC stage. In order to investigate the effects of this additional filtration stage on the toxicity of water and sediment of the receiving water course, samples from four sites at the Schussen River-upstream and downstream of the WWTP Langwiese, prior and after upgrading – were investigated over a period of 2 years by means of the fish embryo test (FET) with the zebrafish (Danio rerio). Additionally, effluent samples from different cleaning stages at the WWTP Langwiese were also investigated with the FET. In summary, our study aimed at assessing the embryotoxic potentials in effluent samples, surface water, and sediment samples of the field sites and, thus, the ecotoxicological benefit of the upgrade of a large WWTP with PAC technology.
The WWTP Langwiese represents a large-sized facility with a cleaning capacity of 170.000 population equivalents. It is located at the Schussen River downstream of the city of Ravensburg (Germany). The WWTP had been a conventional facility (according to the German standard) equipped with a mechanical, a biological, and a chemical purification step followed by sand filtration with combined flocculation as the final cleaning stage. In autumn 2013, it was upgraded with an additional adsorptive PAC stage in order to reduce the concentrations of trace substances in its effluents.
Four field sampling sites were investigated up- and downstream of the WWTP Langwiese (Figure 1). The sampling sites S0 and S1 are situated upstream of the WWTP Langwiese. S0 is also located upstream of the stormwarer overflow basin (SOB) Mariatal which is connected to the WWTP Langwiese. S1 is situated downstream of the SOB Mariatal. The sites S3 and S6 are located 5 km and 17 km downstream of the WWTP Langwiese, respectively. The S6 site is also located downstream of another WWTP at Eriskirch near the Schussen estuary into Lake Constance. Additionally, a reference site (named S4) at the Argen River, another large tributary to Lake Constance, was investigated, since a literature review by Triebskorn & Hetzenauer [25] revealed the Argen River to be a less polluted stream. All sampling dates are listed in Table 1.

Figure 1: Field sampling sites at Schussen River (up- and downstream of the WWTP Langwiese) and at the reference site at Argen River
| Sampling date | Field sites | WWTP Langwiese | |
| Prior to WWTP upgrade |
July 2012 | x | x |
| October 2012 | x | - | |
| May 2013 | x | - | |
| July 2013 | x | x | |
| After WWTP upgrade |
November 2013 | x | x |
| May 2014 | x | x | |
| July 2014 | x | x |
Table 1: Sampling events during the investigation period; x = sampled; - = no sampling
At the WWTP Langwiese, samples from different positions (Figure 2) in the process of sewage cleaning were investigated: a) influent with untreated sewage; b) effluent after biological/ activated sludge treatment; c) effluent of the final purification step (sand filtration and combined flocculation); d) effluent of PAC Filter after upgrade with PAC filter; e) effluent of the final purification step after upgrade with PAC filtration.
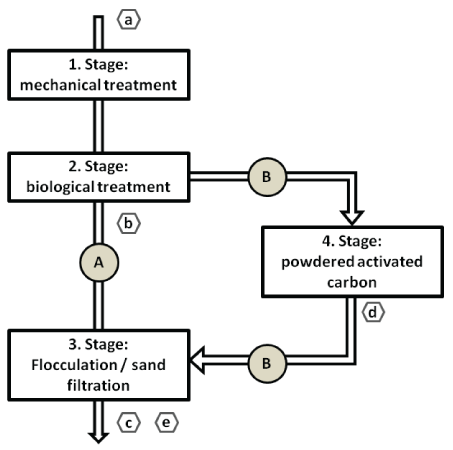
Figure 2: Sampling sites at WWTP Langwies; A: Prior WWTP upgrade; B: After WWTP upgrade. Sampling was conducted at a) influent with untreated sewage; b) effluent after biological/ activated sludge treatment; c) effluent of the final purification step (sand filtration and combined flocculation); d) effluent of PAC Filter after upgrade with PAC filter; e) effluent of the final purification step after upgrade with PAC filtration
Sampling: Sediment and surface water samples were taken at each field sampling site. Sediment sampling was performed close to the riverside, where the top 2 to 4 cm of the riverbed where taken. In order to obtain representative sediment samples of each sampling site, all sediment samples were randomly collected (multiple spots within each investigated field site) over a distance of 15 to 30 meters. Subsequently, the collected samples of each field site were homogenized in a stainless steel bucket and divided into three batches of 100 g, wrapped in aluminum foil (Roth, Germany). Batches were used for the application in the FET. Water was sampled at a depth of 10 to 15 cm from the river surface. All flasks were rinsed with river water before sampling. At each field site and sampling event, three 250 mL sterilized glass flasks (Schott Duran, Germany) were filled with surface water.
Regarding the sampling at the WWTP Langwiese, also three glass flasks (250 mL, Schott Duran, Germany) per sampling event were filled with the corresponding water of each of the investigated cleaning stages. The collected samples represented 24h bulk samples that were taken by installed automatic samplers at each of the investigated cleaning stages. In order to prevent artificial amplification of microorganisms in the samples, they were stored in a cool box at 4°C during sampling and transport and subsequently frozen at −20°C after arrival at the laboratory.
Water pH, oxygen concentration, conductivity, and water temperature were measured directly in the stream during each sampling event. For additional measurements 1 L of river water (per sampling site and event) was sampled in a sterile glass flask and transported to the laboratory. In the laboratory, the concentrations of nitrate, nitrite, ammonium, phosphate, chloride were determined photometrically by using tube test kits (NANOCOLOR® tube tests) and a compact filter photometer (Compact photometer PF-12Plus) from Macherey-Nagel (Düren, Germany). Carbonate and total hardness were determined titrimetrically with test kits (MColortest™ ) from Merck (Darmstadt, Germany). All measured data were assessed and evaluated according to the guidance values defined by the German Working Group on Water Issues [26] and the German Act for the Regulation of Surface Waters of 2011 [27].
Chemical analysis of wastewater samples from the WWTPs Langwiese was performed with high-performance liquid chromatography (HPLC, 1200 or 1290 series Agilent Technologies, Waldbronn, Germany) separation coupled to tandem mass spectrometry (API 4000 and 5500 series, AB Sciex, Framingham, USA). Prior analyses samples were preconcentrated with solid phase extraction (SPE). A detailed description of all analytical methods for benzotriazoles, and pharmaceuticals and antibiotics (including SPE protocol, gradient program and analytical columns) can be found in Thellmann et al. [28]. WWTP influent samples were diluted by a factor of ten and effluent samples by a factor of five, respectively, prior SPE.
dult and juvenile zebrafish (Westaquarium strain) were reared and stocked at the Animal Physiological Ecology Section of Tübingen University. All fish were kept in 100-240 L tanks at water temperatures of 26 ± 0.5°C and adequate oxygen supply. The fish tanks contained filtered tap water (AE-2L water filter equipped with an ABL-0240-29 activated carbon filter, 0.3 μm; Reiser, Seligenstadt, Germany). The quality of the filtered tap water was maintained at 8 to 12 °dH total hardness (equivalent to 1.43 to 2.14 mM CaCO3 ), a conductivity of 260-350 μS/cm, and pH 7.4 ± 0.2. Concentrations for nitrite and nitrate were kept below critical values (nitrite 0.025 to 0.1 mg/L; nitrate 1 to 5 mg/L). Every 14 days, 30% of the water volume was exchanged. The tanks were exposed to an artificial dark-light cycle of 12:12 h. Adult zebrafish were fed three times daily (about 3% of fish weight per day) with dry flake food (TetraMin™ , Tetra, Melle, Germany). Additionally, once in a week and prior to spawning events, the fish were fed frozen food (mosquito larvae) to stimulate optimal egg production. In order to prevent unwanted spawning, tanks did not contain any sediment substrates, plants, and decoration. Breeding boxes, which were used for egg production, were positioned at the bottom of the aquaria the evening before spawning was desired. The breeding boxes consisted of plastic trays topped with stainless steel grids (mesh size 1.5 mm). The steel grids allowed the passage of eggs into the trays and protected them against predation by adult zebrafish. In order to stimulate spawning, a green plastic imitation plant was positioned on top of the steel grids.
Fish embryo test: The fish embryo tests generally followed the procedure of the OECD Guideline 236 [29], and were applied and modified as sediment contact assays according to the work of Hollert et al. [30]. Criteria for the validation of the test were the same as described in the OECD Guideline 236.
All samples were collected in triplicates. Therefore, three independent test runs were conducted for each sampling site and event (three tests on different dates). Tests were conducted as described in the work of Thellmann et al. [28]. For each of the tested sampling sites (in the field and at the WWTP Langwiese) five glass Petri dishes (30 mm diameter, Schott Duran, Germany) were filled with the respective sample. For the testing of the field samples, all dishes were filled with 2.5 g of the sediment sample and overlaid with the corresponding surface water from the same sampling site and event. Reconstituted water (according to ISO 7346/3) was used as negative control. At defined time points, lethal and sublethal endpoints (hatching rate; developmental delays, and failures) as well as developmental stages were observed (Table 2) with a stereomicroscope (Stemi 2000-C, Zeiss, Oberkochen, Germany).
| Endpoints | Hours post fertilization (hpf) | |||||||
| 8h | 12h | 24h | 48h | 60h | 72h | 84h | 96h | |
| Mortality/Coagulation* | X | X | X | X | X | X | X | X |
| Hatching | X | X | X | X | ||||
| Developmental retardations | ||||||||
| Epiboly | X | |||||||
| Gastrulation | X | |||||||
| Formation of somites* | X | |||||||
| Tail detachment* | X | |||||||
| Spontaneous movements | X | |||||||
| Eye development | X | |||||||
| Heart rate (beats/min)* | X | |||||||
| Otolith formation | X | |||||||
| Occurrence of melanocytes | X | |||||||
| Developmental failure | ||||||||
| Oedema (heart and yolk) | X | |||||||
| Malformation of eyes | X | X | X | X | ||||
| Tail deformation | X | X | X | X | ||||
| Spinal deformation (Scoliosis) | X | X | X | X | ||||
| Pigmentation failures | X | X | X | X | ||||
Table 2: Observed developmental stages and endpoints during fish embryo test with Danio rerio; indicators of lethality, when absent, are marked with *
Statistical analyses were performed with SAS JMP version 11.0 (SAS Institute GmbH, Böblingen, Germany). For the analyses of data from the investigated sampling sites at the rivers Schussen and Argen, the entire dataset for all sampling events and all test runs were assessed by the Likelihood-ratio test. The entire dataset recorded for the tested effluent samples from the WWTP Langwiese were analyzed by Fisher’s exact test. The significance level was set to α=0.05. To correct for multiple testing, the Holm–Bonferroni method was applied to adjust the significance levels.
Gererally, the recorded data indicated a good ecological condition of both streams according to the guidance values defined by LAWA [26] and the 2011 German Regulation Act for Surface Waters (Table 3) [27]. The only exception from this was the nitrate concentration at each of the investigated Schussen River sites, for which a good ecological condition was not achieved. The mean values of data from all sampling events are summarized in Table 3.
| Prior to upgrade | After upgrade | |||||||||
| S0 | S1 | S3 | S6 | S4 | S0 | S1 | S3 | S6 | S4 | |
| Conductivity [µs/cm] | 651.2 | 633.8 | 639 | 675.8 | 480.4 | 605 | 603.5 | 610.25 | 628.5 | 504.5 |
| Water temperature [°C] | 14.46 | 14.88 | 15.00 | 15.48 | 13.30 | 12.45 | 12.28 | 12.70 | 12.90 | 12.50 |
| O2 saturation [%] | 101.68 | 99 | 101.42 | 90.94 | 103.58 | 103.6 | 99.95 | 98.5 | 90.65 | 106.62 |
| O2 content [mg/L] |
9.88 | 9.50 | 9.87 | 8.73 | 10.24 | 10.48 | 10.23 | 9.95 | 9.21 | 10.29 |
| NH4 -N [mg/L] | 0.050 | 0.052 | 0.037 | 0.051 | 0.028 | 0.035 | 0.045 | 0.038 | 0.053 | 0.035 |
| NO2 -N [mg/L] | 0.021 | 0.024 | 0.021 | 0.027 | 0.008 | 0.019 | 0.019 | 0.019 | 0.019 | 0.009 |
| NO3-N [mg/L] | 3.063 | 2.932 | 3.369 | 3.384 | 0.902 | 2.875 | 2.825 | 3.275 | 3.675 | 0.900 |
| PO4 -P [mg/L] | 0.06 | 0.05 | 0.06 | 0.06 | 0.04 | 0.07 | 0.06 | 0.07 | 0.07 | 0.03 |
| Carbonate hardness [°dH] | 19.80 | 19.60 | 20.20 | 20.00 | 17.00 | 20.25 | 18.50 | 18.50 | 18.50 | 16.50 |
| Overall hardness [°dH] | 21.00 | 20.80 | 20.20 | 20.20 | 18.80 | 21.75 | 21.50 | 21.75 | 21.50 | 18.25 |
| pH | 8.23 | 8.24 | 8.22 | 8.16 | 8.26 | 8.22 | 8.21 | 8.26 | 8.02 | 8.25 |
| Chloride [mg/L] | 23.40 | 23.40 | 26.00 | 27.80 | 13.00 | 21.75 | 22.25 | 24.00 | 30.00 | 9.50 |
Table 3: Physicochemical water parameters at each of the six field sites, prior to and after the upgrade of the WWTP Langwiese in Ravensburg. Data represent mean values of all conducted measurements. All measured values were assessed and evaluated according to the guidance values defined by LAWA (GermanWorking Group forWater Issues; LUBW2008) and the German Regulation for Surface Waters of 2011 (OGewV 2011). Blue marked values point to very good ecological conditions, green marked values point to good ecological conditions, while orange marked arrays indicate that a good ecological condition is not achieved
Chemical Analyses: Within the frame of the Schussen Aktivplus project, chemical analyses were conducted for more than 100 common substances. In this work, only the data recorded for six frequently occurring substances (1H-benzotriazole, 4-methyl benzotriazole, 5-methyl benzotriazole, carbamazepine, diclofenac and sulfamethoxazole) are presented in the Figures 3-5 and Table 4. These substances were selected as representatives for all anthropogenically introduced compounds. Data presented in the Figures 3-4 and in Table 4 clearly show that the concentrations of all these substances decreased in the effluent and in the field due to the use of the additional PAC stage. The additional elimination rates varied between 59 and 91% depending on the respective substance (Table 4). A summary of measurement data and elimination rates is given in Table 4. At sampling site 6, which is located downstream of the WWTP Eriskirch, a decrease of the concentrations was only observed for three substances (1H-benzotriazole, 5-methyl benzotriazole, sulfamethoxazole), whereas the concentrations for carpamazepine and diclofenac increased after the upgrade of the WWTP Langwiese (Figure 5).
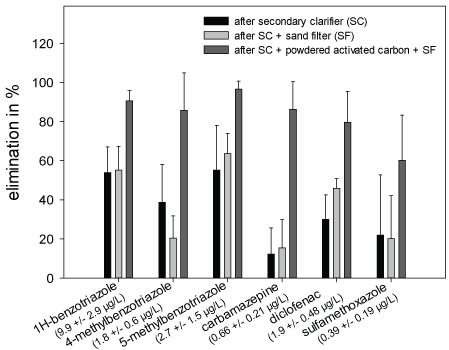
Figure 3: Removal rates of six micropollutants in the WTTP Langwiese after activated sludge treatment (AST), AST + sand filtration (SF), and AST + powdered activated carbon and SF. Values in parentheses indicate concentration in the WWTP influent
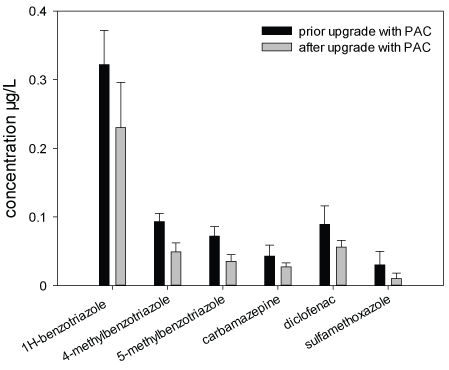
Figure 4: Concentrations of six micropollutants in surface water samples from the Schussen River; sampled at sampling site 3 (downstream of the WWTP Langwiese)
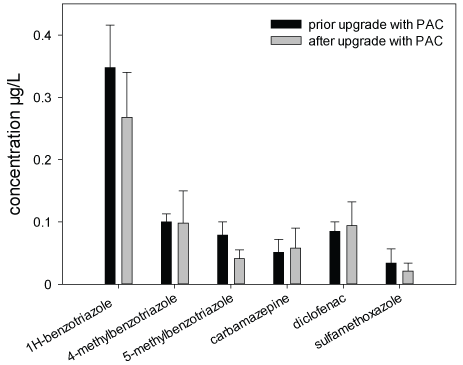
Figure 5: Concentrations of six micropollutants in surface water samples from the Schussen River; sampled at sampling site 6 (downstream of the WWTP Eriskirch and WWTP Langwiese) near estuary into Lake Constance
| Influent | Effluent SC |
Effluent SC + SF |
Effluent SC +SF + PAC |
Additional elimination by PAC [%] | |
| 1H-benzotriazole | 9.90 | 4.30 | 4.40 | 0.95 | 78.41 |
| 4-methyl benzotriazole | 1.80 | 1.20 | 1.60 | 0.25 | 84.38 |
| 5-methyl benzotriazole | 2.7 | 0.9 | 0.89 | 0.08 | 91.01 |
| carbamezepine | 0.66 | 0.55 | 0.56 | 0.09 | 83.93 |
| diclofenac | 1.9 | 1.3 | 0.91 | 0.37 | 59.34 |
| sulfamethoxazole | 0.39 | 0.27 | 0.34 | 0.13 | 61.76 |
Table 4: Measured concentrations (in µg/L) and elimination rates (%) of 1H-benzotriazole, 4-methyl benzotriazole, 5-methyl benzotriazole, carbamazepine and diclofenac in the influent and the effluent of different purification steps of the WWTP Langwiese prior to and after the upgrade with an additional PAC stage. Abbreviations: SC= secondary clarifier; SF= sand filter; PAC= powdered activated carbon
Fish Embryo Test: Only low embryotoxic potentials were found in the samples from the investigated WWTP effluents prior and after the WWTP upgrade. With regard to the mortality rate of the exposed zebrafish embryos, no differences were found between the investigated effluents and the control treatment. Concerning the sublethal endpoints of the test, only low rates of developmental delays (lack of tail detachment) and developmental failures (edema, spinal deformations) were observed. Both, the developmental failure rate and the rate of developmental delays, were similar to those from embryos of the control treatment. The hatching rate showed the most prominent differences between the investigated effluents. Here, a steady increase of the hatching rate from the secondary clarifier up to the final purification step (upgraded with PAC) was apparent (Figure 6). The results also demonstrate that the variability of zebrafish embryo responses decreased in consequence of sewage cleaning by PAC.
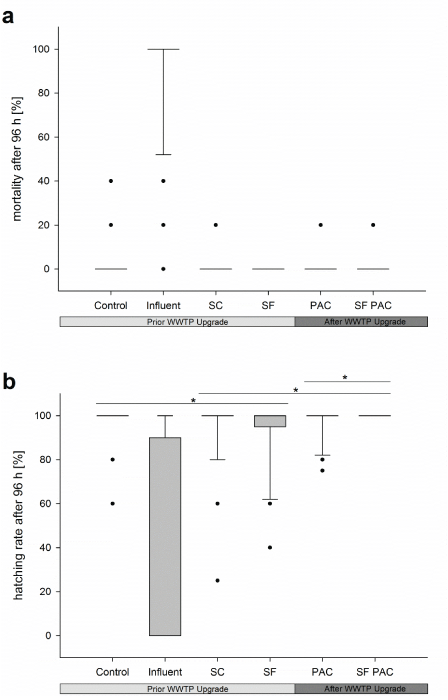
Figure 6: Mortality rates (a) and hatching rates (b) of zebrafish embryos exposed to samples from different effluents of the WWTP Langwiese. Significant differences (*p< 0.05) were found between (1) SC and control treatment; (2) SC and SF PAC; (3) PAC and SF PAC. The entire dataset recorded for all sampling events and test runs were assessed by Fisher’s exact test. In order to correct for multiple testing, the Holm–Bonferroni method was applied. Abbreviations: SC= secondary clarifier; SF= sand filter; PAC= activated carbon; PAC SF = powdered activated carbon followed by sand filtration
The investigation of native sediment and surface water samples from the field sites led to more clear results in respect to the benefit of the additional purification step with PAC. For both endpoints, the mortality and the hatching rate, a significant improvement was observed for the sampling sites S3 and S6 downstream of the WWTP Langwiese. Samples from these sites, taken after the upgrade of the WWTP, resulted in significantly lower mortality rates and significantly elevated hatching rates in exposed embryos compared to those samples that have been tested before the upgrade (Figure 7). The opposite was apparent for embryos exposed to samples from sampling sites 0, 1, and 4. In these cases, sediment and surface water samples taken after the upgrade of the WWTP Langwiese, respectively after autumn 2013, resulted in significantly elevated mortality rates and significantly reduced hatching rates in exposed embryos compared to the corresponding samples that were tested prior to the upgrade (Figure 6). Except for site 0, samples taken from all sites exerted a significantly lower developmental delay rate after the upgrade of the WWTP Langwiese in autumn 2013 than corresponding samples have done before.
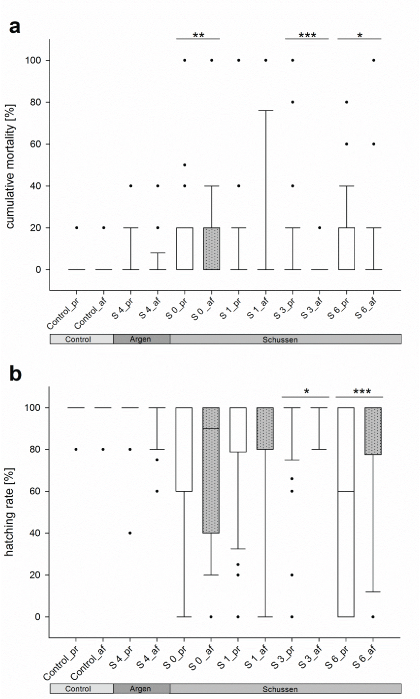
Figure 7: Mortality rates (a) and hatching rates (b) of zebrafish embryos exposed to native sediment and surface water samples from the rivers Schussen and Argen. Significant differences (*p<0.05; **p<0.01; ***p<0.001) were found at sampling sites 0, 3, and 6 at the Schussen River. The entirety of data for all sampling events and test runs were assessed by the Likelihood ratio test. Treatment description: treatments ending with _b = prior to WWTP upgrade with PAC; treatments ending with _a = after WWTP upgrade with PAC
The physicochemical measurements did not reveal any differences between the investigated sampling sites. With exception of the elevated nitrate concentrations at the Schussen River, all measured values indicated a good ecological condition according to the guidance values defined by the German Working Group on Water Issues (LAWA) [26] and the German Regulation Act for Surface Waters of 2011 [27] The elevated nitrate concentrations at the Schussen River can likely be attributed to humic substances which occur naturally in the Schussen River and also to intense agricultural land use in the Schussen catchment area. Nevertheless, it was obvious that differences in zebrafish development as recorded in the FETs on environmental samples cannot be reasonably attributed to these physicochemical parameters.
Data from the chemical analyses clearly demonstrate that the additional purification step with PAC results in a further reduction of the measured chemical substances in both the effluent of the WWTP Langwiese and in samples of the sampling sites 3 and 6 (Table 4 and Figures 3-5). However, at sampling site 6, the concentrations for two compounds (diclofenac and carbamazepine) were slightly increased after the upgrade of the WWTP Langwiese. This increase can mainly be explained by the discharges of the WWTP Eriskirch, which is has not been upgraded with an additional cleaning stage. The obtained data are particularly important with regard to frequently used compounds like carbamazepine, benzotriazole, diclofenac, and many other substances which are not or only insignificantly reduced during conventional waste water treatment, even in WWTPs equipped with 3 purification steps. With regard to the reduction of trace substances and toxic effects, the effectivity of an additional PAC stage in waste water treatment (pilot- and full-scale studies) has been previously described in several studies such as, e.g., Boehler et al. [31], Margot et al. [21], Altmann et al. [32], or Mailler et al. [33]. All of these studies highlighted the importance of the additional purification step for a sufficient and sustainable waste water treatment. However, studies addressing the impact of such WWTP upgrades on the toxic potential in compartments of connected ecosystems are rare.
Studies on effluent samples from the WWTP Langwiese revealed only minor effects on zebrafish embryogenesis. Even though mortality did not differ significantly between conventional and advanced treatment, with regard to sublethal endpoints an elevated hatching rate in embryos exposed to PAC-treated wastewater was observed. This result points to a slight but still recognizable further reduction of embryotoxic potentials due to the use of the additional PAC-based purification step. In this context it needs to be considered that the ‘conventional’ treatment steps in a technologically highly developed country (tertiary treatment with sand filtration and combined flocculation) already resulted in a distinct reduction of embryotoxic potentials during the purification process. Nevertheless, our data show, even at this high technological level, that a further reduction of embryotoxic potentials in effluents can be achieved by PAC-technology.
In addition, fish embryo tests with native sediment and surface water samples from the investigated field sites revealed even clearer effects. Here, a significant reduction of embryotoxic effects was observed in samples of the sampling sites 3 und 6 (downstream of the WWTP), after the upgrade of the WWTP Langwiese, which is particularly indicative for the improvement at these downstream locations as the sampling sites 0, 1 (upstream of the WWTP), and 4 (reference site) that are not influenced by this WWTP showed the opposite trend at the same time. For this reason, the improvement of the situation at sites 3 and 6 cannot result from a general fluctuation of environmental parameters from year to year but rather has to be related to the specific location of these two downstream sites. Sediments provide a large number of binding sites for several contaminants due to their composition by inorganic and organic components [34,35]. Thus, they are able to temporally integrate over the toxicity exerted by pollutant burdens over a longer time span. They also deliver more information about pollution levels than a 24h bulk sample taken from a WWTP effluent. The chemical analytics identified several substances like the widely used pharmaceuticals diclofenac and carbamezepine in the effluent of the WWTP Langwiese. Many of the substances originating from municipal wastewaters are known for their lipophilicity and low degradability in the environment and, therefore, are able to accumulate in stream sediments [36-38]. A general statement about the accumulation or biodegradation of substances in the environment cannot be made, as both parameters depend on many factors like pH, charge of the compound, total organic carbon (TOC) and oxygen conditions [39,40]. Furthermore, most of the sediments in European rivers - including the Schussen River - already contain persistent and harmful substances like PAHs, PBDEs, and also abandoned compounds like PCBs [41,42] which interact with the effects of substances deriving from current waste water release. In the past years a number of studies have been published that demonstrated the effects of the above-mentioned substances on the health and the development of exposed zebrafish embryos. For instance, Perrichon et al. [43] exposed zebrafish embroys to fluoranthene-spiked sediments and observed increased mortality rates and various developmental alterations. Similar observations were made by Usenko et al. [44] in zebrafish embryos exposed to different PBDE congeners. However, it is documented that these compounds which have been identified as priority pollutants often only count for a minor part of the biological response [45,46]. A recent study of Qiang et al. [47] showed that even low and environmentally relevant concentrations of carbamazepine are able to affect zebrafish development on the molecular level. In this study, already 1 µg/L carbamazepine impaired the expression pattern of neural-related genes in zebrafish embryos. Schwaiger et al. [8] exposed rainbow trout (Oncorhynchus mykiss) to different concentrations of diclofenac. The authors observed alterations in gills and kidney of rainbow trout after exposure to diclofenac in the lower µg/L range. Similar observations were made by Hoeger et al. [48] after exposure of brown trout (Salmo trutta f. fario) to different and environmentally relevant concentrations of diclofenac. The above-mentioned examples and also many other studies demonstrate that numerous anthropogenic substances which are released into rivers by WWTPs are able to affect not only the health and the development of the model organism zebrafish, but also the health of feral organisms. Since it is impossible to cover the entirety of pollutants present in the environment by chemical analytics, it cannot be decided which of these substances finally contributed to the observed effects in our experiments. Therefore, the studied effects have to be attributed to the entirety of factors present in water and sediment, including mixture effects and interactions of chemicals with confounding factors. In this case, the fish embryo test with the zebrafish and native sediment and surface water samples represents an adequate research method since it integrates over the impact of all compounds present in an environmental sample.
The obtained results revealed a positive development of the ecotoxicological situation at sites 3 and 6, downstream of the WWTP effluent. This positive effect can likely be attributed to the upgrade of the WWTP with an additional PAC stage in autumn 2013, because the WWTP Langwiese is, by far, the largest sewage treatment plant at the Schussen River and its released waste waters represent 50 percent of the total wastewater load in this stream. It is noteworthy that this significant reduction of effects in samples of sites 3 and 6 after the upgrade of the WWTP Langwiese was visible despite the presence of 17 other, smaller WWTPs upstream of the WWTP Langwiese releasing their effluents into the Schussen River. The efficiency of this WWTP upgrade with a PAC stage was also demonstrated by Maier et al. [49] and Peschke et al. [50] who observed a distinct reduction of dioxin-like potentials in the WWTP effluents and an improvement of invertebrate’s health and diversity in the Schussen River, respectively. In general, the positive long-term effects of WWTP upgrading with powdered activated-carbon was demonstrated in the works of Triebskorn et al. [51] and Thellmann et al. [28]. The river Schmiecha, also located in Southwest Germany, which has been in the focus of the aforementioned studies, was historically highly polluted by waste waters released from textile industry. The stream was reported to appear ‘stained in all colours’ in these days, and higher organisms were not able to survive in the polluted water [28]. In order to reduce the toxicity of effluents from the local textile industry, the connected WWTP in Albstadt-Ebingen (Schmiecha River) was equipped with a PAC stage and combined flocculation more than 20 years ago. Measurement data of Vogel et al. [52] have proven an effective reduction of micropollutants by the powdered activated carbon stage in the WWTP Albstadt-Ebingen. The upgrade with the additional PAC stage also resulted in a highly efficient recovery of the ecosystem as shown in the works of Thellmann et al. [28] and Triebskorn et al. [51].
Our results clearly demonstrate that an additional cleaning stage based on powdered activated carbon (PAC) represents an efficient and adequate technology for the reduction of trace substances in the treated waste water and also, notably, for the reduction of embryotoxic potentials in stream sediments. Activated carbon filtration is an advanced technology which takes advantage of the adsorption of contaminants onto a large inner surface of PAC that is between 300 und 2000 m² per gram, due to the high porosity of the particles. Adsorption of substances to PAC, particularly organic chemicals that may exert embryotoxicity, but also other chemicals like chlorine or fluorine is based on van der Waals forces. This method has been shown to be very effective in removing organic chemicals in high concentrations, e.g. dyes, from wastewater [53]. The positive effects of the additional PAC stage on indigenous fish and invertebrates has been demonstrated in the works of Henneberg and Triebskorn [18], Maier et al., [49] and Peschke et al., [50]. Our results indicate that this additional wastewater treatment technology is not only of high relevance for the sustainable protection of aquatic biota, but is also of high relevance for humans. The latter is particularly the case when surface waters are used for drinking water supply. With regard to the demands of the EU Water Framework Directive (WFD), advanced waste water treatment with powdered activated carbon has to be regarded as an efficient technology for the sustainable protection of surface waters and aquatic biota. Furthermore, the FET with the zebrafish (Danio rerio) applied to native surface water and sediment samples proved to be a useful tool to assess the impact of WWTP effluents on the ecotoxicology of connected streams.
The authors want to thank Hans Güde (ISF Langenargen), Sabrina Giebner (Frankfurt U), Hans-J. Vogel (Regional Council Tübingen), Andreas Dieterich, Anja Henneberg, Stefanie Krais (all Tübingen U), and all other colleagues for their support during the sampling campaigns.
The project Schussen Aktivplus was funded by the German Federal Ministry for Education and Research (BMBF) and co-founded by the Ministry for the Environment, Baden-Württemberg. The project was connected to the BMBF action plan “Sustainable water management (NaWaM)” and was integrated in the BMBF frame programme “Research for sustainable development FONA”. Contract period: 1/2012 to 12/2014, Funding number: 02WRS1281A. Furthermore, financial contribution has been provided by Jedele & Partner GmbH, the AZV Mariatal, the city of Ravensburg, Ökonsult GbR, and the AV Unteres Schussental.
Also, we acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
The authors declare that there are no competing interests. The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
Download Provisional PDF Here
Article Type: Research Article
Citation: Thellmann P, Greiner-Perth K, Jacob S, Knoll M, Schäfer M, et al. (2017) Does Waste Water Treatment Plant Upgrading with Powdered Activated Carbon Result in Reduced Water and Sediment Toxicity of the Receiving Stream? Int Water Wastewater Treat 3(2): doi http://dx.doi.org/10.16966/2381-5299.141
Copyright: © 2017 Thellmann P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access