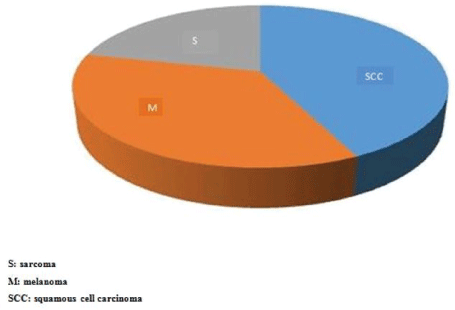
Figure 1: Distribution of patients by histological types of primary tumors.
Sidy KA1* Abdourahmane N Dong2 Mamadou M Dieng3 Jaafar Thiam1 Adja Coumba Diallo1 Doudou Diouf1 Macoumba Gaye4 Ahmadou Dem5
1Joliot Curie Cancer Institute, Dakar, Senegal*Corresponding author: Sidy KA, Joliot Curie Cancer Institute, Dakar, Senegal, Tel: +221338250530; E-mail: sidy.ka@ucad.edu.sn
Background: Inguinal lymph node dissection helps to prevent groin and systemic metastasis during surgery for limb and lower genitourinary cancers. The objective of this work was to report the indications, techniques and results of inguinal lymph node dissection in cancers of the lower limb at the Dakar Cancer Institute.
Methods: This was a retrospective study over a 7-year period. Included patients were over 15 years of age and had lower limb cancer regardless of the tissue involved. The parameters studied were age, gender, histological type, lymph node involvement, postoperative morbidity, recurrence and survival.
Results: There were 70 patients operated over a period of 7 years. The average age of our patients was 53.4 years. The sex ratio was 0.94 with 34 men and 36 women. The most common histological type was squamous cell carcinoma with 30 cases (42.8%). There were more clinical and histological involved nodes in the case of sarcomas. Histological findings were done in 26 of 54 patients (48%). We noted 3 cases of death (4.2%) in a context of kidney failure for 1 case and respiratory distress 2 cases. The local complications consisted of suture release in 2 cases, 1 operative wound necrosis, 1 amputation wound suppuration. After five years of follow up, no patients had chronic sequels following inguinal dissection, 5 patients (7.7%) had local recurrence, 4 patients (7.7%) had node metastases and 1 patient developed liver metastases. We have recorded 20 deaths (28.6%) related to cancer.
Conclusions: Inguinal lymph node dissection showed good results in limb cancer treatment. Squamous cell carcinoma is the main histologic cancer and lymph involvement is more frequent in case of sarcomas. Complications are rare and death occurred mostly for related to cancer stage.
Groin; Lymph node; Metastasis; Morbidity; Mortality
Inguinal lymph node dissection is done in principle to detect lymph node involvement and remove metastatic nodal disease. Most of these lesions are secondary localization of cutaneous cancers, soft tissue sarcomas and osteosarcomas of the lower limb [1,2]. Its prognostic value is not always well established and it is responsible for many immediate and chronic complications [3]. The objective of this work was to report the indications, techniques and results of inguinal lymph node dissection for lower limb cancers at Joliot Curie Cancer Institute.
This was a retrospective study over a 7-year period. Included patients were over 15 years of age and had lower limb cancer regardless of the tissue involved. The parameters studied were age, gender, histological type, lymph node involvement, operative morbidity, recurrence and survival.
There were 70 patients operated over a period of 7 years. The average age of our patients was 53.4 years with extremes of 17 and 78 years. The sex ratio was 0.94 with 34 men and 36 women. The most common histological type was squamous cell carcinoma with 30 cases (42.8%), followed by acralmelanoma in 25 cases (35.7%) and sarcomas in 15 cases (21.4%) (Figure 1). At the time of diagnosis, 52 patients (74.3%) had palpable lymph nodes. Malignant fungus was present in 15 of 52 patients with palpable lymph nodes. There were more clinical (Table 1) and histologically (Table 2) involved nodes in the case of sarcomas. Histologic findings were done in 26 of 54 patients (48%) (Figure 2). There was no vascular and nerve injury reported. The average duration of hospitalization was 5 days with extremes of 3 and 40 days. The local complications consisted of suture release in 2 cases, 1 operative wound necrosis, 1 amputation wound suppuration. Post operative death occurred in 3 cases (4.2%), 1 in a context of kidney failure and 2 in a context of respiratory distress. After five years follow up, no patients had chronic sequels following inguinal dissection, 5 patients (7.7%) had local recurrence, 4 patients (7.7%) had node metastases and 1 patient developed liver metastases. We have recorded 20 deaths (28.6%) related to cancer.

Figure 1: Distribution of patients by histological types of primary tumors.
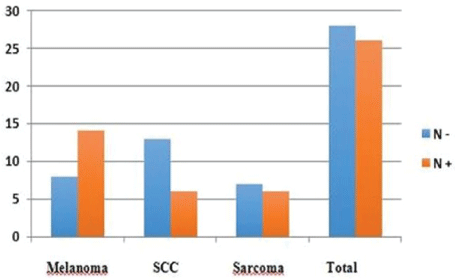
Figure 2: Histologic node involvement.
| Histological type | Non palpable nodes | palpable nodes |
| Melanoma | 6 (24%) | 19 (76%) |
| SCC | 9 (30%) | 21 (70%) |
| Sarcoma | 3 (20%) | 12 (80%) |
| Total | 18 (25.7%) | 52 (74.3%) |
Table 1: Clinical node involvement according to histological type.
| Histological type | N+ | N- |
| Melanoma | 14 | 8 |
| SCC | 6 | 13 |
| Sarcoma | 6 | 7 |
| Total | 26 | 28 |
Table 2: Histologic node involvement according to cancer type.
The average age of occurrence of cancers of the lower limb is 50 years. The average age of inguinal dissection according to authors depends to the histological type ranging from 20 to 60 years for sarcomas to more than 70 years for the squamous cell carcinomas [4-6]. The sex ratio varies according to the histological type with a general trend in favor of men [7,8].
Clinical node involvement is variable. Melanoma is more likely to involve lymph nodes than other histological types. Due to the delay in consultation the majority of our patients already had a clinical ganglionic extension at their first consultation. In case of extra-ganglionic metastasis, lymph node dissection is highly recommended [9,10]. The histologic lymph node involvement is also variable according to the histological type [2]. The capsular effraction and the number of affected nodes are important prognostic factors especially in melanoma. The inguinal clearance limited to the groin or extended to the iliac area according to the involvement or not of the Cloquet node remains unclear [5,8]. Squamous cell carcinoma and sarcomas regardless the stages are not likely to affect inguinal ganglions [11]. Morbidity is close to 70% of cases of inguinal dissection and there is considerable variation between authors [2,12]. There is a high frequency of postoperative complications like disruption of sutures, skin necrosis, seroma and early lymphedema. The oblique incisions are less deleterious than the vertical incisions [12]. Mortality directly related to inguinal dissection as well as postoperative mortality is very low. It is related to the comorbidities and to the underlying cancer [13]. Recurrences are mostly observed at the primary cancer site. It is very rare after inguinal clearing except for the big attacks and malignant fungus [14]. Although rare in our series, the most common chronic complication is lymphoedema. It is more frequent after radiotherapy of the limb or groin [12]. Many methods are increasingly being used for its evaluation, prevention and treatment [15]. It uses endoscopic and micro-invasive surgical techniques, drainage, dissection and physiotherapy. However, the results are not formal [3]. Whether prophylactic or performed after sentinel node biopsy, inguinal lymph node dissection has not shown significant benefit in terms of overall survival. However, it can prevent a disabling inguinal disease [16].
Clinical and histological inguinal node involvement is more frequent in our practice than in the literature. This justifies a maximalist therapeutic attitude to prevent inguinal recurrences. Chronic complications including lymphoedema are underestimated and require better evaluation methods for prevention and treatment. It is important to sensitize populations to help for early diagnosis of lower limb cancers to optimize the outcomes of their management.
None
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Ka S, Dong AN, Mamadou MD, Thiam J, Diallo AC, et al. (2018) Inguinal Lymph Node Dissection for Lower Limb Cancer: Indications, Technics and Results about 70 Cases. J Surg Open Access 4(3): dx.doi. org/10.16966/2470-0991.161
Copyright: © 2018 Ka S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access