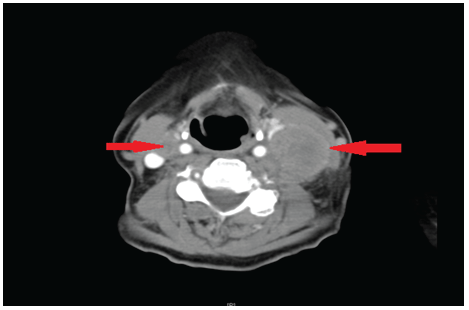
Figure 1: CT neck with contrast indicating bilateral lymphadenopathy (arrows).
Rajesh Anmolsingh1* Rachel Edmiston1,2 Anisa Ali3 Paul W Bishop4 Anshuman Chaturvedi4 Vijay Pothula1
1Department of ENT, Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK*Corresponding author: Rajesh Anmolsingh, Department of ENT, Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK, E-mail: ranmolsingh@yahoo.com
Neuroendocrine tumours (NET) of the head and neck are a rare diverse group of tumours. In 2005 World Health Organisation (WHO) Classification of Head and Neck tumours lists 4 types of Neuroendocrine carcinomas: (a) typical carcinoid (b) atypical carcinoid, (c) small cell carcinoma, neuroendocrine type and (d) combined small cell carcinoma, neuroendocrine type with non-small cell carcinoma. There has been considerable debate about non-inclusion of large cell neuro endocrine carcinoma and need for further classification with inclusion of different terminology. We report a case of squamous cell carcinoma of the tongue base with bilateral neck nodes containing NET differentiation of small cell on one side and a large cell on the other. No such case is reported in the literature so far.
Small cell NETs are reported in the larynx, sino-nasal tract, salivary glands, trachea, oral cavity and oropharynx [1,2]. Large cell NET is also described in the oropharynx [3], parotid gland [4], larynx [5] and hypopharynx [6]. Primary tumours in the head and neck have been reported, which have combined Neuroendocrine and Squamous cell carcinoma in the maxillary sinus [7] and larynx [8].
Neuroendocrine tumours in primary head and neck regions are known to metastasis to neck nodes. Hatoum et al. [1] suggested 80% of known disease stage, presented with cervical lymphadenopathy at diagnosis. Mills et al. [9] reported a cumulative incidence of 93% cervical lymphadenopathy for laryngeal NET. Even large cell NETs are believed to metastasis to cervical lymph nodes [3].
Case Report
A 69-year-old lady, who was referred by the haematology department, presented with a neck lump of 2-3 weeks’ duration. She gave no history of dysphagia, hoarseness of voice, loss of weight or referred pain in the throat or ear. She had no history of any other serious illnesses or operations beside an appendicectomy. Family history revealed her son died of testicular cancer
Neck examination elicited a large lump at level 3-4 on the left side and palpable right level 2 lymph nodes. ENT examination including fibre optic examination showed no obvious primary tumour. CT scan (Figure 1) showed bilateral neck nodes with prominent soft tissue at the base of tongue. Fine needle aspiration for cytology showed small cell carcinoma with a differential diagnosis of possible lymphoma. She subsequently underwent an incisional biopsy of left neck lump which was reported as metastatic small cell carcinoma. She underwent a PET scan which highlighted increased uptake on right base of tongue and bilateral neck nodes. After discussion in multi-disciplinary team meeting, the patient underwent bilateral neck dissection (Figures 2-4) and radiotherapy to her tongue base. The radiotherapy regime involved Intensity-modulated radiotherapy (IMRT) at a dose of 66 Gy in 33 fractions, 6 fractions per week, over 6 weeks. Three years after surgery, she remained tumour free in the head and neck region. On regular monitoring, approximately 38 months after bilateral neck dissection, the patient developed haemoptysis and investigations in the form of a CT scan revealed a primary lung tumour with multiple mediastinal metastases. The histology from core biopsy of the lung tumour showed non-small cell carcinoma with no histological evidence resembling head and neck pathology. She is currently on palliation.

Figure 1: CT neck with contrast indicating bilateral lymphadenopathy (arrows).
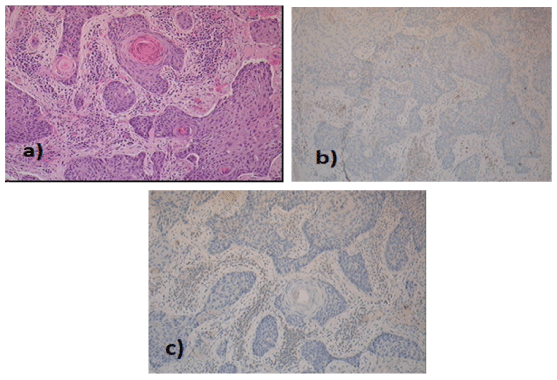
Figure 2: Histology of base of tongue (magnification 10x) a) well-differentiated keratinising squamous cell carcinoma b) CD56 and c) synaptophysin negative.
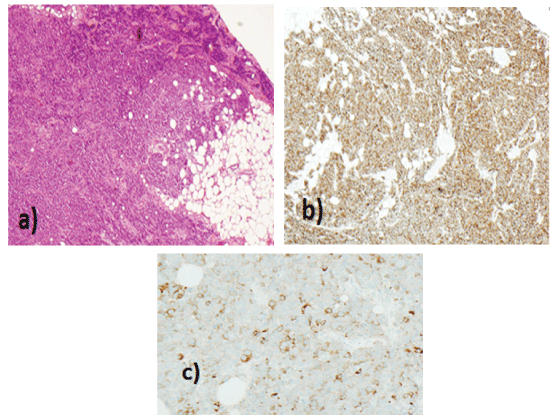
Figure 3: Histology of Left Level III neck node (magnification 10x) a) high-grade neuroendocrine carcinoma more ‘small cell type appearances b)CD56 and c) synaptophysin positive.
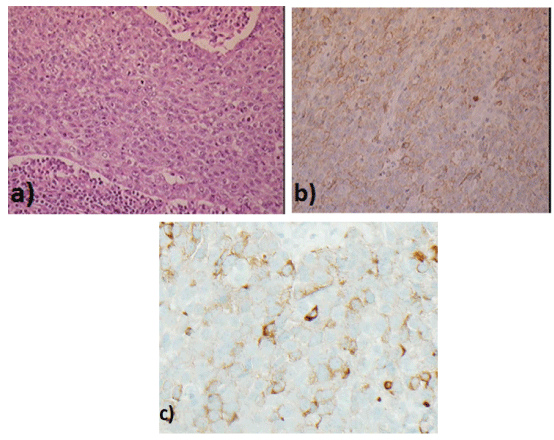
Figure 4: Histology of Right neck node (magnification 10x) a) high-grade neuroendocrine carcinoma more ‘small cell type appearances b) CD56 positive c) synaptophysin positive.
Neuroendocrine neoplasms, defined as epithelial neoplasms with predominant neuroendocrine differentiation, arise in most organs of the body [10,11]. Conceptually, it is the secretion of bioactive substances (e.g. hormones, bioamines) by neoplastic cells [12]. They are detected using tumour markers such as Chromografin A and synaptophysin; though the role of somatostatin receptor has also been statistically significant in the detection of neuroendocrine tumours [13].
Poorly differentiated neuroendocrine tumours (PD-NET) are highgrade carcinomas that exhibit neuroendocrine differentiation [12]. PDNET are further classified into small cell carcinomas, large cell carcinomas and mixed.
Neuroendocrine differentiation has been found in some tumours not considered to be of neuroendocrine origin including squamous cell carcinoma of the lung and oesophagus [13,14]. All the cases reported in the literature with metastatic neck nodes containing NET, have their primary NET located in the head and neck region. Our case has a primary squamous cell carcinoma with metastatic neuro endocrine tumour. The other unusual feature of this case is the tumour on the left side contains small cell carcinoma and on the right side of the neck showed large cell features both showing features of neuroendocrine differentiation with no squamous cell features.
In the literature, there is a case in larynx reported with squamous cell cancer on one side and NET on the other [8] and a metastatic neuro endocrine tumour in the neck node presenting as unknown primary but laryngeal primary was detected 8-10 months later [15].
Neuroendocrine features (established by immunohistochemistry or electron microscopy) may be present in otherwise conventional carcinomas with a classically described non- neuroendocrine nonsmall cell morphology (including adenocarcinomas and squamous cell carcinomas) and in such instances, at non-head and neck sites, the presence of this morphology has further been suggested to have prognostic implications [16].
Carcinogenesis is considered to incorporate cancer stem-cell driven and clonal evolutionary processes [17]. Head and neck cancer studies have led to the discovery of a small population of cancer ‘stem’ cells (CSC), the majority being found within a 100 μm-radius of a blood vessel - indicating existence of a probable perivascular niche, that behave as tumour progenitor cells (including exhibiting self-renewal and multipotency) and potentially play a role in chemotherapy resistance and also in “driving” recurrence and metastasis [18,19]. The role of such pluri-/multipotent cancer stem cells within tumours has been suggested as a mechanism for divergent or trans-differentiation to other cell lineages such as neuroendocrine differentiation in an otherwise morphologically primary squamous cell carcinoma – as seen in our patient [20]. More recent research in stemcell research suggests that this ability of CSC to transdifferentiate into cell lineages other than the original lineage from which the carcinoma arose may also possibly offer new therapeutic strategies [21]. In the present case, there was an indication that scanty early metastatic carcinoma cells lay dormant within lymph nodes before finally being identified as distinct clinical/ radiological metastatic masses. Such a scenario has previously been well covered in literature [22,23].
Some cancer stem cells may respond to radiotherapy based on tumor biomarkers and HPV status [24,25]. The patient was fortunate enough to have complete resolution of tongue base tumour. More recently there has been a change in practice to radiotherapy with concomitant chemotherapy for the more difficult malignancies which are believed to have higher counts cancer stem cells or less favourable biomarkers.
Oropharyngeal tumours have high association of HPV [15,26]. Presence of HPV in SCC of Oropharynx demonstrated improved outcomes [26]. This favourable prognosis is absent in oropharyngeal NETs despite positivity to HPV [27]. In our case neither the primary squamous cell carcinoma of base of the tongue nor the metastatic NET neck nodes have shown any positivity to HPV.
Gorner et al. [28] have reported 10 patients - 6 of whom are poorly differentiated NET and have died within 2 years with multimodality treatment and 4 are free of disease, 3 of which are paranasal sinus NETs and one larynx. Ferilito et al. [29] reported 16% 2-year survival.
Conclusions
Contrary to the belief that tumours with neuro endocrine differentiation are believed to be aggressive and have poor prognosis, our case survived for 3 years without any local recurrence. Unfortunately, she presented with a second primary in the lung 3 years later which is a non-small cell cancer. This case is unique since the primary tumour was squamous cell carcinoma with metastatic nodes showing neuroendocrine differentiation. The learning point is that cancer ‘stem’ cells have the potential to differentiate into different histological presentations.
The authors declare no conflict of interests.
Download Provisional PDF Here
Article Type: Case Report
Citation: Anmolsingh R, Edmiston R, Ali A, Bishop PW, Chaturvedi A, et al. (2017) An Unsusual Extrapulmonary Neuroendocrine Tumour of Head and Neck. J Surg Open Access 3(3): doi http:// dx.doi.org/10.16966/2470-0991.153
Copyright: © 2017 Anmolsingh R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access