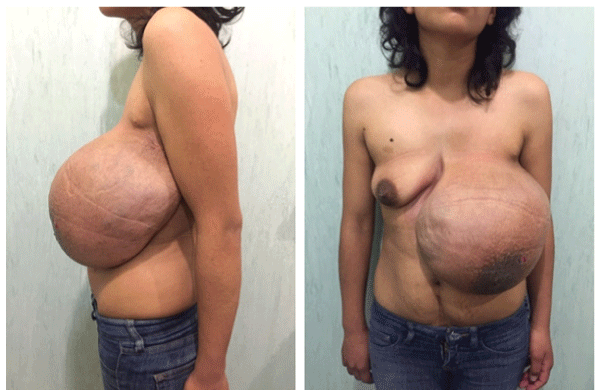
Figure 1: Tumor involving the entire left breast, dimensions of 20 × 30 cm. Skin color change due to venous dilation at surface, no nipple discharge or changes.
Carlos Daniel Gómez-Calvo1 Ilich Sandino Ríos-García2 Héctor Bizueto-Rosas1* Hugo Alonso Pérez-González3 Luisa Fernanda Hernández-Rivera1 Rafael Aburto-Pérez1 Rafael Armenta-López1
1Vascular Surgery and Angiology Department, Specialties Hospital, “La Raza” National Medical Center, Mexican Institute of Social Security, Ciudad de México, Mexico*Corresponding author: Héctor Bizueto-Rosas, Vascular Surgery and Angiology Department, Specialties Hospital, “La Raza” National Medical Center, Mexican Institute of Social Security, Juárez, Mexico, Tel: (55) 3599 0728; E-mail: dr_bizueto_h@yahoo.com
Background: Cystic lymphangioma is a rare congenital anomaly that affects children under two years of age. In adults, it´s even more rare, especially in an axillary location. It is related to trauma, infection and has an iatrogenic cause.
Case: We report the case of a 26-year-old woman with a history of a left hemithorax hemangioma at age 12, with multiple previous interventions, that at 25 years old, presents sudden growth of the lesion associated with pregnancy. The CT scan ruled out a deep tissue and contralateral breast compromise, so we performed resection with mammoplasty and nipple-areola replantation altogether with the Plastic Surgery department. She was discharged with a closed suction drain. The histopathology conclusion was of cystic lymphangioma and lymph fluid without malignancy data.
Conclusions: The diagnosis and treatment of these lesions must be done early, take a multidisciplinary approach and surgical resection is recommended. The MRI imaging is the preferred study of choice.
Breast tumor; Cystic lymphangioma; Axillary lymphangioma
We report the case of a 26 years old female, with no significant family history. Previous medical history of right femur osteosynthesis due to fracture secondary to vehicle-pedestrian accident at age 6, requiring blood transfusions at the time. Began her current condition at age 12 when she noticed an increased volume in the outer upper quadrant of the left breast, having hemangioma as the established diagnosis at the time.
General Surgery performed a resection of the lesion; six months later, she noticed new volume increase and underwent resection again. She remained asymptomatic until six years ago, when she presents volume increase of the lesion again, this time it was removed by the Plastic and Reconstructive Surgery department (PRSD).
Two years later, presents a new relapse being re-intervened by PRSD, with apparent good evolution until last year in which, after pregnancy, presents an important volume increase, rapid growth and this time within durated consistency and venous dilation for which was referred to our service with suspicion of vascular anomaly.
At physical examination was found with adequate nutritional status, without jugular engorgement or axillary lymphadenopathy; old surgical scars in anterior axillary line and third left intercostal space. A tumor of 20 × 30 cm involving the left breast, with venous dilatations on its surface, soft, non-painful, partial renitent, mobile, without nipple discharge, no changes in temperature and murmurs of thrill (Figure 1).

Figure 1: Tumor involving the entire left breast, dimensions of 20 × 30 cm. Skin color change due to venous dilation at surface, no nipple discharge or changes.
CT thoracic angiogram was performed (we did not have access to MRI), and it reported a significant volume increase, with measures of 24.5 × 20.8 cm in maximum diameter, at the expense of multiple lesions with irregular morphology, having 5 Hounsfield Units (HU) single phase densities, homogeneous (Figure 2).

Figure 2: Tumor of 24.5 × 20.8 cms, with multiple homogeneous lesions, irregular morphology, no contrast capitation and no vascular or deep tissues involvement.
Arterial and venous phases were obtained without showing signs of direct vascular involvement; in arterial phase, the lesions had 19 HU and in the venous phase had 39 HU. At the edges of the tumor, small vascular tracts were observed, with no affection of the contralateral breast. The radiological diagnosis was hemangioma (Figure 2).
Due to complex tumor architecture, no vascular involvement, several previous surgical procedures and benign behavior history over years of multiple relapses surgical excision with definitive histopathology examination was offered as treatment.
It was performed surgical resection altogether with the PRSD, having a complete resection of the lesion and dissection towards the axillary space and below the pectoral muscle. The lesion could be identified in the suprafacial and muscular plane of the thoracic wall; after incision 3000 ml of chalky pink chylous material was obtained, there were lymphatic pathways that came from the axillary region and the axillary vein, which was dissected by isolating the lymphatic vessels, with ligation of that appeared to be a vascular pedicle. There was no evidence of infiltration of neurovascular structures. Once the lesion was resected, we proceed to do a Wise-pattern mastectomy with breast reconstruction and nipple-areola re-implantation and placement of a closed surgical drain (Figure 3).
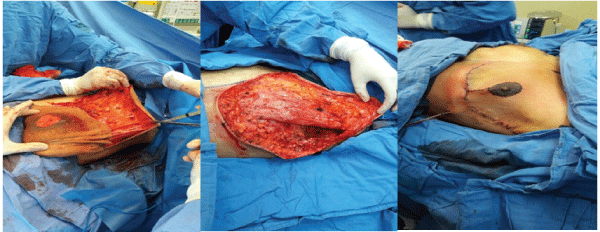
Figure 3: The lesion was suprafascial; resection and axillary dissection was initially performed and a Wise-pattern mastectomy with breast reconstruction and nipple-areola re-implantation afterwards.
PRSD plans to use a sub-pectoral pocket silicone implant for breast reconstruction after a year in order to reduce the risk of relapse. The patient had a satisfactory evolution, with no wound complications and no surgical-site infection (Figure 4).
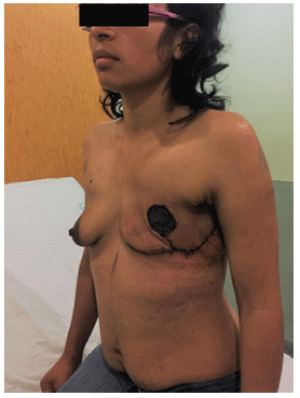
Figure 4: Three weeks after surgery. No surgical site infection and no signs of early relapse with good aesthetical evolution.
The histopathological study and fluid analysis conclusion were of cystic lymphangioma: “Great cysts surrounded by endothelium, collagen and muscle fibers, lymph, fat and mammal areola tissue. No cellular mitosis observed. Fluid obtained was suggestive of lymph, without malignancy data”.
The cystic lymphangioma (CL) or cystic hygroma (CH) occur because of an incomplete development of the lymphatic vessels [1]. It develops mainly during childhood and represents the 6% of the soft tissue tumors in children [1]. It is benign, rarely occurs in the adulthood and it is even rarest in an axillary location [1-3] they are produced by congenital or acquired lymphatic vessels anomalies [4]. It grows slowly and painless; occasionally they can have sudden growth [5] or present involution [4].
There are three histological varieties of lymphangiomas accordingly to the tissue in which they settle: capillary, characterized by thin capillary wall; cavernous (the most frequent) where the muscular and fibrous tissue predominate, and cystic, composed of delimitated cysts by an endothelial layer of variable size, presented in lax tissue locations that can reach a great size [6]. The lymphangiomas of the mammary gland are very rare and their main location is at the external upper quadrant, this due to lymphatic drainage of the gland (Spence’s tail and axillary region) [7].
At diagnosing, ultrasound is useful because it can show either the solid or cystic nature [2,8]. The computerized tomography scan (CT scan) can determine the extension, compromise of surrounding structures and can show if a multiloculated cystic tumor is present, usually with septa that are enhanced after using contrast [2].
Fine needle aspiration (FNA) guided by ultrasound is used to rule out malignancy and can ratify the diagnosis by obtaining proteinaceous and lymphocytic material and to be able to perform AAFB and inflammatory response studies [2]. Magnetic Resonance Imaging (MRI) is the study of choice to determine the extension and involvement of deep structures [2]. The differential diagnosis it´s made with lipomas, soft tissue sarcomas and breast dependent tumors with axillary involvement [7].
Burezq et al. [8] mentions that percutaneous aspiration is an appropriate management. Sclerosing agents such as OK432 can be injected usually after aspiration and emptying of the tumor; these agents diffuse into the stroma, causing irritation, inflammation, scar retraction and contraction of the lesion [9].
The OK432 developed in1986 is obtained from a lyophilized mixture of Streptococcus pyogenes of group A type III (loses its antigenicity when incubated with penicillin G and results in the complete disappearance of streptolysin S). It is sclerosing due to its immunomodulatory activity, induces killer cell activity and can cause high fever; its maximum dose is 0.2 mg in 20 ml of saline solution. It should not be given to patients allergic to penicillin [9].
For great size lesions surgical resection with the attachment of a fibrin adhesive layer (Tissucol®) and closed surgical drainage is the most effective method of choice [7,8]. Some authors believe that small cystic lymphangiomas should not be operated, but kept under observation and further treatment depends on their size and location [7]. Relapses have been associated with pregnancy and lactation [10]. Laparoscopy approach is useful when recurrent retroperitoneal cystic lymphangiomas are found; it is often presented because of difficult dissection and incomplete previous resection [11].
Vascular lesions, such as lymphatic vascular malformations, are the most frequent [12]. It is very common to confuse them with venous lesions and thereby delay treatment and mismanagement, with the dire consequences observed in the clinical practice by delaying their surgical removal [8]. According to the Hamburg classification, which divides them into two groups, troncular and extra-troncular, cystic lymphangiomas of hygromas, are more common in children and usually manifest before 2 years of age12.
In the case of adults, they are more frequent in women and the axillary location is very rare. These lesions are painless, slow growing and sometimes grow suddenly after a history of infection, trauma or iatrogenic. Adult development is proposed to be due to delayed cell proliferation; however, there are usually predisposing factors [7].
Lymphangiomas of the mammary gland may be aggressive locally but are benign lesions [12]. Some authors consider that lymphangiomas are hamartomatous malformations secondary to the inability of the lymphatic system to communicate with the venous system. Other authors are of the opinion that cystic lymphangiomas, as mentioned above, according to the Hamburg classification, represent the lymphatic tissue sequestered that cannot be normally communicated with the lymphatic system (extratroncular), so that, in the absence of communications with some trunk, they form large sacs that accumulate important amounts of liquid and that proliferate to the surrounding tissues [12].
The axillary location in the adult is asymptomatic, and to the physical examination, cystic, well defined and frequently progressive. CT scan should be contrast enhanced to determine if there are septa and to differentiate it from soft tissue sarcomas. Radionuclide studies serve to delimit the drainage source [4].
Vargas-Hernández et al. [7] make a highly detailed differential diagnosis of histological findings to differentiate these lesions from cavernous hemangiomas, a pathology that is very often confused with lymphangiomas since we often forget that lymphatic are the most frequent vascular malformations. In the histopathological study can be identified lymphatic channels with blood content for secondary hemorrhage that can cause an erroneous diagnosis such as cavernous hemangioma, but, evidencing collections of lymphoid cells in the stroma, and sometimes lymphoid follicles formation and irregularity of the cavernous spaces, confirm the diagnosis of lymphangioma [13].
Endothelial vascular markers (VIII factor and an antigen associated with CD31) may be positive in the lymphatic endothelium, but they do not serve to establish the differential diagnosis of hemangioma and lymphangioma [13].
The definitive diagnosis is made with the histopathological study; Laminin can be expressed in the basal lamina of lymphatic vessels, but it does better in the blood vessels and makes a clear distinction between the two structures [13]. Regarding treatment, good results have been reported in 97% with the administration of OK432 and simple aspiration, however, in masses of large volume, for obvious reasons, such amount of sclerosant cannot be administered by toxic effects.
The recurrence of lymphangioma after treatment with OK-432 according to Luzzato et al. [14] is 11%, they even recommend it as presurgery treatment, because of the complex and often incomplete removal of the tumor, with higher rates of recurrence and persistent symptomatic disease; although OK-432 it’s difficult to find in some countries. However, today the total resection of lesion treatment is the one of choice; in addition, it is the only way in which the correct diagnosis can be established. It is probable that in our case, the lesion originated in the axillary region and extended to the mammary gland since the patient does not remember very well the clinical scenario and has ancient scars in the anterior axillary line. To our knowledge these cases are very rare, up to 2001 there were only six reported cases plus the one reported by Vargas-Hernández in 2014.
Conclusions
It is a priority to keep in mind that these lesions (Lymphatic) are the most frequent, to establish a diagnosis and proper management. Multidisciplinary treatment and early surgical removal are indispensable. “Think not about the relapse risk, but in the patient quality-life” Individualizing each patient is mandatory
The authors declare no conflicts of interest
Download Provisional PDF Here
Article Type: Case Report
Citation: Gómez-Calvo CD, Ríos-García IS, BizuetoRosas H, Pérez-González HA, Hernández-Rivera LF, et al. (2017) Breast Giant Cystic Lymphangioma with An Axillary Origin. A Case Report. J Surg Open Access 3(2): doi http://dx.doi.org/10.16966/2470- 0991.147
Copyright: © 2017 Gómez-Calvo CD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access