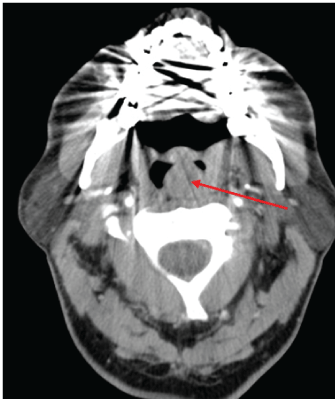
Figure 1: CT image demonstrating a 17 mm, round lesion lying left of the midline arising from the posterior wall of the oropharynx.
R Anmolsingh1 B Yu1 O Mirza1 J Rocke1 B Ranganathan1 A Ismail2 BN Kumar1*
1Department of ENT, Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK*Corresponding author: B Nirmal Kumar, Department of ENT, Wrightington, Wigan and Leigh NHS Foundation Trust, UK, E-mail: nirmalkumar@doctors.org.uk
Myoepithelioma is an extremely rare tumour subtype and diagnosis is based on a wide variation of cellular morphology. They are poorly characterised in terms of their clinical behaviour, histopathological appearance and immunochemical profile, which makes their diagnosis and the onward management of a patient challenging. We present a case where the lesion was initially thought to be a paraganglioma, but following a second histological opinion was found to be a myoepithelioma. To the best of our knowledge, this is the first reported case of a myoepithelioma arising from the posterior oropharyngeal wall.
Myoepithelioma; Oropharyngeal wall; Tumour
Myoepitheliomas are rare salivary gland tumours which make up between 1 and 1.5% of all salivary gland tumours. They are most commonly found in the parotid gland and palatal area [1]. Historically, they were initially classed as a subtype of pleomorphic adenoma, but were recognised as a separate tumour class by the World Health Organisation in 1991. Myoepitheliomas, however, continue to remain poorly characterised in terms of their clinical behaviour, histopathological appearance and immunochemical profile, which makes their diagnosis and the onward clinical management challenging [2].
We present a case which illustrates the ongoing difficulty in diagnosing such tumours and the impact of differential diagnoses with a range of outcomes and prognoses on a patient’s management plan. To the best of our knowledge, this is the first reported case of a myoepithelioma arising from the posterior oropharyngeal wall.
A 56-year-old man presented electively to ENT outpatients with a few week history of a painless posterior oropharyngeal wall swelling. The swelling had increased in size over several weeks and had started to cause some post-nasal obstruction and subsequent snoring. There were no other concerning upper aerodigestive red flag symptoms. He had a background of psoriasis, but no other medical comorbidities. He was an ex-smoker with a moderate alcohol intake. Examination revealed a 2 cm nonulcerated, pedunculated mass arising from the posterior oropharyngeal wall at the level of the soft palate. The rest of the pharynx and larynx were normal. There was no palpable cervical lymphadenopathy. A decision was made to proceed with surgical excision, once imaging had been carried out.
A contrast CT scan was performed to further characterise the lesion (Figure 1). This showed a 17 mm, round well defined, thin walled lesion lying left of the midline, at the level of the tonsils arising from the posterior wall of the oropharynx. No evidence of surrounding invasion was seen and the prevertebral fat plane was intact. Radiological features were suggestive of either a solid mass or a cystic fluid filled lesion. An MRI was considered to further characterise the soft tissue mass, however, this was contraindicated in our patient due to implanted metal.

Figure 1: CT image demonstrating a 17 mm, round lesion lying left of the midline arising from the posterior wall of the oropharynx.
Complete trans-oral excision of the lesion was performed and the lesion sent for histological analysis. Histology (Figures 2a-2c) showed a completely excised well-circumscribed tumour. The tumour was composed of zell batten-like nests, clusters and irregular bands of uniform tumour cells with indistinct cytoplasm and limited pleomorphism. The tumour cell aggregates were separated by a sclerotic vascularised stroma. There was no capsule, spindle cells, splayed nerve fibres, hyalinised blood vessels or necrosis. Only rare mitosis was seen. Immunohistochemical staining was positive for S100 (Figure 3a), GFAP (Figure 3b), CD56, synatophysin, NSE, CD-99, bcl-2 and pGP9.5. Staining was negative for vimentin, desmin, SMA, CD 14, Melan-A, Neu-N, CD 117, HMB-45, DOG1, AE1/AE3. There was no evidence of malignancy but nuclear Ki67 labelling index of 5-8% was in the range of aggressive tumour behaviour.

Figure 2:
2a: H&E × 5 magnification showing a well demarcated tumour.
2b: H&E × 10 magnification. Tumour cells in solid trabeculae with round to spindle shaped nuclei.
2c: H&E × 40 magnification. Tumour cells are uniform with clear to amphophilic cytoplasm.
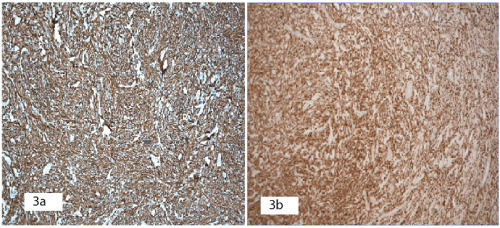
Figure 3: Immunohistochemistry staining.
3a: S100 protein: Positive.
3b: GFAP: Positive.
Based on this, there was some uncertainty with regards to the final histological and immunochemical findings. Although, the immunophenotypic characteristics were unusual, the findings were suggestive of a neuroendocrine neoplasia best conforming to an aggressive paraganglioma.
The patient was followed up four weeks following surgical excision. He had made an uneventful recovery. However, on clinical examination there was a small residual lesion at the site of surgical excision. Due to the histological report suggestive of a paraganglioma and a strong clinical suspicion of reoccurrence at the original tumour site, despite previous complete clinical and histological excision, a repeat CT scan was organised and the patient was discussed at both the head and neck cancer and neuroendocrine cancer multi-disciplinary team (MDT) meetings.
A repeat contrast CT showed some mild asymmetrical thickening along the left posterior oropharyngeal wall at the site of the previously resected lesion which could represent either post-surgical changes or residual tumour (Figure 4). There was no local or distance evidence of metastatic spread and no paraganglioma evident along the sympathetic chain.
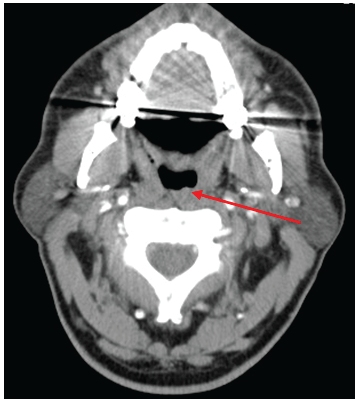
Figure 4: A repeat postoperative CT image demonstrating a mild thickening along the left posterior oropharyngeal wall at the site of the previously resected lesion, likely representing either post-surgical changes or residual tumour.
Due to the histological indecision, the MDT suggested a second review of the specimen should be sought. A second opinion described only a vague nesting pattern of cells, where well developed nests are expected for a paraganglioma, which were not a prominent feature. Furthermore, the cells also had patchy staining for vimentin which had previously been reported as negative. Vimentin together with S-100 are sensitive but not specific markers for neoplastic myoepithelium. Overall it was concluded that the findings were not supportive of a paraganglioma, but more suggestive of a benign myoepithelioma, perhaps of a minor salivary gland.
Re-excision of the residual lesion was performed and reassuringly the histology simply showed some reactive granulation tissue, with no evidence of residual myoepithelioma or neoplasia (Figure 5). No further treatment was necessary. The patient was followed up 4 months later, with no evidence of reoccurrence and remains under regular review.
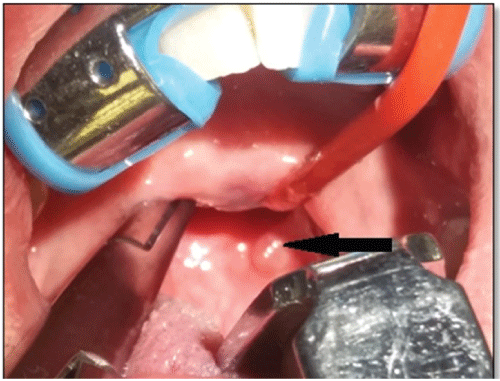
Figure 5: Intraoperative clinical photograph with Boyle Davis mouth gag and soft palate/uvula retracted, demonstrating a residual left oropharyngeal lesion (arrow).
Both paragangliomas and myoepitheliomas are rare head and neck tumours which can be identified clinically or found incidentally radiographically. They typically present asymptomatically as painless slow growing lesions, but neither characteristically arises in the oropharynx. Imaging is often non-specific, and not usually helpful in the diagnostic process. Diagnosis can be challenging due to an inherent wide variation of cellular morphology, and therefore relies heavily on histology and immunohistochemistry of the lesion.
Myoepithelial cells are present in major and minor salivary glands, sweat glands, lacrimal glands, prostate, breast, nasopharynx, lung, retroperitoneal, skin and soft tissue [3]. Myoepitheliomas develop preferentially in the parotid gland. Minor salivary glands follow in frequency, especially in hard and soft palate. Submandibular gland, sublingual gland and other minor salivary glands can also be affected. Three-fourths of all cases of myoepitheliomas occur in the parotid gland and palate [3]. The recurrence rate is 10% and, although rare, malignant transformation (myoepithelial carcinoma) can occur [4]. Paragangliomas are neuroendocrine tumours which account for 0.6% of all head and neck tumours [5]. They are most commonly found in the carotid body, jugular tympanic cavity and cervical vagus nerve. The majority are benign, but around 3% can be malignant and able to produce distant metastases. Local recurrence rate is 17% and may be a sign of malignancy. For both lesions surgical excision is the mainstay of treatment, whilst radiotherapy can also be used concurrently with surgery or as a single modality treatment in certain situations, such as a confirmed malignant tumour, multi-site paraganglioma or patients unsuitable for surgery [6]. Close and prolonged follow up is recommended.
The uncertainty in the diagnosis of myoepithelioma is due to the wide range of benign and malignant differential diagnoses which exist, depending on the predominant cell type [7].
Immunohistochemistry is therefore important in the process of making a firm diagnosis for both tumour types and to distinguish them from other differentials. However, myoepitheliomas have a complex histomorphology and variable expression of antigenic markers. Myoepitheliomas can exhibit a combination of the four main cell morphologies: spindle, epithelioid, plasmacytoid and clear cells. They can also display variable growth patterns: nonmyxoid, myxoid, reticular, or mixed. This can be attributed to the various stages in the differentiation from a cell that has the potential to differentiate into epithelial cells [3]. Vimentin and S-100 protein are very sensitive, but non-specific, markers of neoplastic myoepithelium. As neoplastic transformation of myoepithelial cells can result in loss or modification of their smooth muscle phenotype, variable positivity for calponin, smooth muscle actin, muscle specific actin, smooth muscle myosin P63, glial fibrillary acidic protein, and CD10 can be seen [7].
Our case highlights the diagnostic challenge inherent in identifying and managing these lesions. The lesion was located in the oropharynx, a location where myoepitheliomas usually do not arise. Although initially thought to be a paraganglioma, a myoepithelioma was only confirmed following a second histological review. Myoepitheliomas are rare and rely on histological appraisal through the recognition of a wide range of morphological features and immunohistochemical profiles in order to facilitate identification. This can be challenging and we, therefore, recommend an MDT approach and suggest the consideration of a second histological opinion if diagnostic uncertainty exists.
Download Provisional PDF Here
Article Type: Case Report
Citation: Anmolsingh R, Yu B, Mirza O, Rocke J, Ranganathan B, et al. (2017) Myoepithelioma of the Posterior Oropharyngeal Wall: A Difficult Diagnosis. J Surg Open Access 3(2): doi http:// dx.doi.org/10.16966/2470-0991.141
Copyright: © 2017 Anmolsingh R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access