Abstract
Objective: Suvorexant, a dual orexin receptor antagonist, was launched as a hypnotic in Japan in 2014. We summarize our experience with this drug at our facilities and clarify the group of patients with effectiveness.
Methods: The study was conducted from January 1, 2015 to December 31, 2015. 60 patients with insomnia were included. Subjects were 44 outpatients and 16 inpatients. Most of the patients were prescribed suvorexant in addition to existing sleeping pills.
Result and Conclusions: Suvorexant was effective for wake after sleep onset (WASO, 85%) as compared with difficulty getting in sleep (74%). Although some cases presented hangover effect (17%), there was no case that caused serious side effects. Half dose tablets (i.e., 7.5 mg or 10 mg) could reduce the hangover effect. If the patients could not sleep well within 30 min after taking medication, suvorexant could improve effectiveness by 60-90 min administration before going to bed. Considering each disease group, there was no therapeutic effect on delirium, however, evaluations of preventive effect would be necessary to be further evaluated.
Since, suvorexant worked well on patients with alcohol-related disorder group, this medicine would be suitable for cases which crosstolerance between alcohol and GABA-A positive allosteric modulators exists, because suvorexant does not interact with GABA-A receptor systems.
No effects on type 1 narcolepsy patients complaining about WASO were observed, and no exacerbations of symptoms with REM related and daytime sleepiness were recognized. We can conclude that suvorexant are suitable for elderly patients, because there are few side effects such as muscle relaxation and rebound insomnia. Since elderly subjects often have WASO than difficulty getting to sleep, it is also appropriate for this medication for these subjects.
Keywords
Suvorexant; Sleep disorder; Wake after sleep onset; Alcohol-related disorder
Abbreviations
WASO: Wake After Sleep Onset; BZ: Benzodiazepine.
Introduction
In 1998, it was discovered as a pair of similar neuropeptides, orexin-A and orexin-B [1]. Orexin-producing cells are present in the lateral hypothalamic area and have the effect of awakening by projecting to the ascending reticular activating system. Almorexant was the first dual orexin receptor antagonist to be developed. Subsequently, suvorexant was launched in Japan ahead of the rest of the world in 2014.
In many cases, GABA-A allosteric modulators (benzodiazepine (BZ), non-BZ) were often used as hypnotics. However, these drugs have side effects including muscle relaxant action, withdrawal symptoms, and dependence [2]. In addition, these drugs also disturb physiological sleep architecture and suppresses deep slow wave sleep and REM sleep [2]. For these reasons, it is difficult to feel refreshed with these drugs. Meanwhile, since the action mechanism of suvorexant is different from GABA-A allosteric modulators, there is little influence on physiological sleep architecture [2]. So far, there are reports on evaluating drug efficacy and side effects for healthy subjects and insomnia patients [3-5] and reports on safety to patients with a sleep apnea [6,7]. It has also been reported in clinical trials that suvorexant works for sleep onset and sleep maintenance for patients with primary insomnia [8,9]. In Japan, suvorexant has been reported to have efficacy for patients with REM related behavior disorder [10], overeating at midnight [11], circadian rhythm disorder [12], bruxism [13], and insomnia in child and adolescent [14].
However, few studies investigate about what types of patients/ sleep disorders should be prescribed suvorexant. Therefore, in this study, we summarize prescription experience at our facilities to highlight the group of patients with high effectiveness.
Methods
The study was conducted from January 1, 2015 to December 31, 2015. Of the 65 patients with insomnia visited the center of psychosomatic medicine, Akita Red Cross Hospital, department of psychiatry, Yuri Kumiai General Hospital and Akita University Hospital, 60 subjects who could be traced after suvorexant prescription were included. 45% of the subjects were under 65 years old and 55% were 65 years old or over (mean age 62, distribution: 16-91 years old, male/female: 27/33). 44 were outpatients and 16 were inpatients. Most of the patients (56 patients) were prescribed suvorexant in addition to sleeping pills they normally take. 33 (55%) patients had difficulty getting to sleep, 28 (47%) patients complained about wake after sleep onset (WASO), and 5 patients had both complaints.
Primary diseases were as follows: 17 cases of primary insomnia, 13 cases of mood disorders, 10 cases of physical disorders, 7 cases of dementia, 4 cases of alcohol-related disorders, 3 cases of schizophrenia, 2 cases of eating disorders, 2 cases of narcolepsy type 1, 1 case of anxiety disorder and 1 case of PTSD (Figure 1). We also prescribed suvorexant in 8 cases of delirium. The initial dose was 20 mg under the age of 65 and 15 mg at age 65 or over, due to the approved dose by the ministry of health in Japan. Evaluation of efficacy was examined every 2 to 4 weeks and evaluated to 3 levels (improvement, unchanged, discontinued) based on subjective assessment by clinical impressions. In the cases with delirium, evaluation of efficacy was examined every week. Because suvorexant is not recommended to administer narcolepsy patients, 2 narcolepsy cases were not included in calculation and statistical analysis.
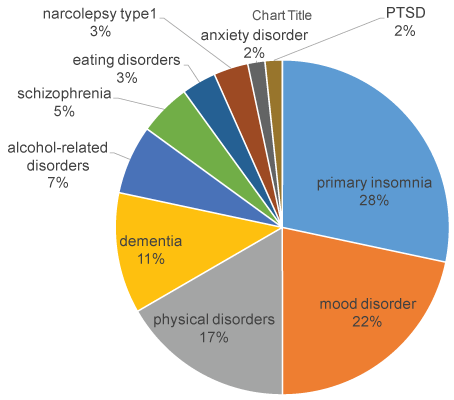
Figure 1: Distribution of primary disorders.
Subjects had the following primary disorders: 17 cases of primary insomnia, 13 cases of mood disorders, 10 cases of physical disorders, 7 cases of dementia, 4 cases of alcohol-related disorders, 3 cases of schizophrenia, 2 cases of eating disorders, 2 cases of narcolepsy type1, 1 case of anxiety disorder, 1 case of PTSD.
This study was approved by ethics committees of Akita Red Cross Hospital, Yuri Kumiai General Hospital and Akita University Hospital, and it is conformed to the provisions of the Declaration of Helsinki.
Results
The outline of the effectiveness and adverse effects
In the 42 outpatients, 30 patients improved either difficulty getting to sleep or WASO, 4 cases were unchanged and 8 cases discontinued. In 16 inpatients, 8 cases were improved, 1 case was unchanged and 7 cases discontinued (4 cases were delirium patients). Adverse effects were reported in 13 cases; 10 felt hung over (dosage was reduced in 5 cases), 1 experienced dizziness, 1 experienced headache and 1 experienced dry mouth. In the 10 discontinued cases, 8 cases did not improve their difficulties getting to sleep (most of these cases took medication 30 min before going to bed).
Effects for getting to sleep and WASO
According to the type of insomnia (WASO or difficulty getting to sleep), suvorexant improved symptoms in 85% of patients complaining WASO and 74% of patients complaining difficulty getting to sleep (Figure 2). Medication was discontinued in 4% of patients with WASO and 21% of patients with difficulty getting to sleep. From these results, suvorexant seems suitable for patients suffering from WASO. On the other hand, even in cases of difficulty getting to sleep, we found cases in which symptoms improved greatly by oral administration 60-90 mins before bedtime. These cases showed that the timing of medication affects the action of suvorexant.
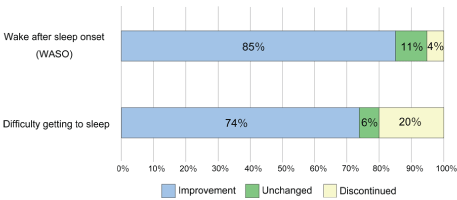
Figure 2: Efficacy of suvorexant depends on type of sleep disorders.
Considering the type of insomnia and effectiveness, improvement of symptoms was observed in 85% of wake after sleep onset (WASO) and 74% of difficulty getting to sleep. In addition, 4% of patients with WASO and 21% of patients with difficulty falling asleep discontinued the medication. In the cases having both symptoms, we asked them about the improvements of each condition.
Dose adjustments of suvorexant
We analyzed the final dose of suvorexant for each age group. In patients under 65 years old, 20 mg was 33%, 15 mg was 57% and 10 mg was 10%. In patients over the age 65, 20 mg was 4%, 15 mg was 72%, 10 mg was 8% and 7.5 mg was 16% (Figure 3). In 15 cases in which administration was discontinued, 4 patients who did not reduce the dosage complained about the hangover. Since the medication was only available in 20 mg and 15 mg tablets in Japan, during latter half of this study, we recommended the patients to cut and only take half of the medication, if they experienced hangover effects.
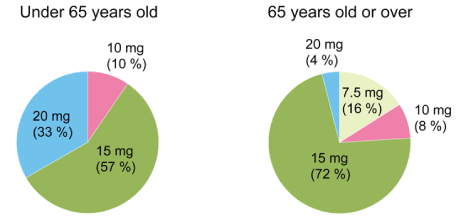
Figure 3: The final dose of suvorexant.
We analyzed the final dose of suvorexant depending on the age groups. In patients under 65 years old, 20 mg was 33%, 15 mg was 57% and 10 mg was 10%. In patients 65 years old or over, 20 mg was 4%, 15 mg was 72%, 10 mg was 8% and 7.5 mg was 16%. Since the medication was only available in 20 mg and 15 mg tablets in Japan, we recommended the patients to cut and only take half of the medication, if they experienced hangover effects.
Alcohol-related sleep disorders
In sleep disorders complicated with alcohol-related disorders group, symptoms improved in all 4 cases. This suggests that suvorexant would be suitable for cases which cross-tolerance between alcohol and GABA-Amodulators exists because it has a different mechanism of action.
Effects to delirium
We also examined the effectiveness of suvorexant on delirium. 8 patients (37-91 years old, median 74.5 years old) consulted for delirium from other departments were included. The diagnosis of delirium was in accordance with the diagnostic criteria of DSM-5, and the initial dose was 15 mg in all cases. Underlying medical conditions were adhesive intestinal obstruction due to resection of rectal cancer, pancreatitis, left femoral trochanter fracture, acute respiratory distress syndrome, purulent spondylitis, multiple trauma, and burn injury. Among them, only 3 patients showed improvement 1-2 weeks after administration, and the efficacy rate was 38%.From these results, the effectiveness of suvorexanton delirium seems to be insufficient.
Insomnia symptoms with narcolepsy patients
Suvorexant was prescribed for two patients with narcolepsy type1 complaining about WASO. The compound did not have any main nor adverse effects.
Discussion
Half-life (t 1/2) of suvorexant, type of insomnias and dose of medication
According to pharmacokinetic data of suvorexant, its t1/2 is about 12 hours and Cmax is about 2 hours [14]. From the viewpoint of the needed receptor occupancy, it is about 8 hours to maintain the blood concentration to occupy 65% of orexin receptor [14].
That is, the effect of the medication gradually appears and is considerably long lasting. It’s effect on WASO observed in this study seems to reflect this pharmacokinetics.
The main reason for discontinuation was that there were no improvements on difficulties falling asleep. Most of these patients took the medication 30 min before going to bed, and they could not sleep soon. During the latter half of this study, we recommended the patients take the medication 60-90 min before going to bed, if they hadn’t been able to sleep well within 30 min of taking medication.
Even in this study, in some cases, suvorexant’s effect was only observed when time of administration was advanced. It is also reported that Tmax is prolonged when suvorexant is administered immediately after a meal [15], so it should not be taken soon after dinner. Suvorexant should also not be taken together with alcohol because it can cause excessive drowsiness. Although no direct comparative trials have been done in this study, the effect for difficulty getting to sleep seems to have no superiority with GABA-A allosteric modulators. On the other hand, the relatively long half-life period is responsible for the hangover effect [5]. In this study, the hangover effect was the most frequent adverse effects, 10 cases had hangover in 13 cases reporting adverse effects.
Since the hangover effects tend to appear in a dose-dependent manner, half of the initial dose (ex. 7.5 mg or 10 mg) would be better to continue administration. 15 mg and 20 mg tablets are available in Japan, but in the United States, 5 mg and 10 mg tablets are also available. Since the starting dose in the United States is 10 mg, 15-20 mg tablets may be too high for Japanese patients. For this reason, we concluded that half tablets should be prescribed for hangover cases. In fact, we experienced that use of half-tablets reduced hangover effects and improved sleep hygiene.
Fortunately, there were no cases of serious adverse effects such as muscle relaxation and rebound insomnia. GABA-A agonists had problems with muscle relaxation and ataxia particularly in elderly patients. On the other hand, suvorexant have less muscle relaxation effect and is thus suitable for elderly patients.
Since elderly subjects more often have WASO than difficulty falling asleep, this medication is also appropriate for these subjects.
For example, administering GABA-A agonists to elderly patients harbors high risk for falls at the time of walking for nighttime urination. Suvorexant may be able to reduce this risk. In addition, few rate of rebound insomnia [14] so it would be easy to switch to other hypnotics and discontinue the medication. On the other hand, when replacing ultra-shorttime type or a short-time GABA-A allosteric modulators, we should add suvorexant and decrease these medications gradually in order to prevent rebound insomnia.
Patients with alcohol-related disorders
Patients with alcohol-related disorders are at high risk for sleep disorders [16]. Alcohol and GABA-A allosteric modulators both act on neuronal GABA-A receptors competitively. In patients with high alcohol intake, the GABA-A receptor is down-regulated and the binding site of GABA-A allosteric modulators is decreased [17]. In this study, suvorexant worked well on all 4 patients from the alcohol-related disorder group. Suvorexant, an orexin receptor antagonist, does not mediate the GABA receptor, and is considered to be suitable for patients in the alcohol related disorder group.
Effects for delirium
Delirium is an acute consciousness disorder mainly occurring during hospitalization in 10-40% of hospitalized patients. Delirium become a risk of falls, aspiration pneumonia, and also serious problems such as, pulling out tubes [18]. Therefore, symptomatic relief has been mainly attempted by the use of antipsychotic drugs, but definitive treatment method has not been established. Because suvorexant suppresses the action of orexin, it was speculated to have effects for hyperactive delirium due to hyperarousal. However, in this study, efficacy for delirium was only 38%. For this reason, it is speculated that each delirium cases have variable underlying medical condition and variable clinical presentations state. The dopamine system might contribute to pathophysiology of delirium more importantly than the orexin system. On the other hand, while the preventive effect has been suggested [19], it was ineffective for delirium which has already developed in this study. Recently, Hatta et al. reported the effects for the prevention of delirium by randomized, placebo-controlled clinical trial [20].
Concerning narcolepsy
Since orexin had already disappeared in the patients with orexin-deficient narcolepsy, suvorexant is considered to have no effect for the patients with narcolepsy. Suvorexant use in narcolepsy patient is contraindicated in the USA, and careful administration is practiced in Japan, symptoms can possibly worsen. Our study did not show any improvement in WASO but did not show any exacerbation of symptoms with REM related and daytime sleepiness. However, the cases with narcolepsy type 2 would be worsened by suvorexant, because their orexin levels in cerebrospinal fluid are normal (i.e., orexin non-deficient).
Limitation
We were aware of the limitations of this study. (1) The number of patients was relatively small. (2) The patients were subjectively evaluated only. (3) This study was retrospectively examined without placebo controls. However, few studies have investigated about prescription of suvorexant, therefore, we summarize our experiences and highlight the group of patients in which the drug was effective.
Conclusions
Suvorexant is more effective for WASO than for difficulty falling asleep. Although some cases presented with hangover effects, there were no cases that reported serious side effects. Half tablets (7.5 mg or 10 mg) can reduce the hangover effect. Suvorexant may improve effectiveness for difficulty getting to sleep by oral administration 60-90 min before sleep onset, if the patients could not sleep well within 30 min after taking medication. Considering each disease group, there was no therapeutic effect on delirium, however, evaluation of preventive effect would be necessary. Since suvorexant worked well on the alcohol-related disorder group, this medicine would be suitable for cases in which cross-tolerance between alcohol and GABA-A allosteric modulators exists, because it has a different mechanism of action. No improvement was observed in 2 narcolepsy patients with complaints of WASO, and no exacerbations of symptoms with REM related and daytime sleepiness were recognized.
We conclude that suvorexant is suitable for elderly patients, because there are few side effects such as muscle relaxation and rebound insomnia. Since elderly subjects often have WASO rather than difficulty falling asleep, this medication is also appropriate for these subjects.
Declarations
Ethics approval and consent to participate
This study was approved by ethical committees of Akita Red Cross Hospital, Yuri Kumiai General Hospital and Akita University Hospital, and it is conformed to the provisions of the Declaration of Helsinki.
Consent for publication
The consent for publication was obtained from all individual participants included in this study.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no conflict of interests.




