Abstract
A 72-year-old febrile man was diagnosed as pneumonia, for which he was given oral tosufloxacin and intravenous ceftriaxone. Although Haemophilus influenzae sensitive to fluoroquinolones and ceftriaxone was isolated from his sputum, there was no improvement of his severe cough and fever. Eventually, his symptoms and laboratory data improved after administration of methylprednisolone. These findings suggested that H. influenzae induced immunologic mechanisms and was strongly toxic.
Keywords
Haemophilus Influenzae; Tosufloxacin; Levofloxacin; Ceftriaxone; Methylprednisolone
Introduction
Haemophilus influenzae is a bacteria that can infect people of all ages, with manifestations that range from mild (e.g., ear infection) to severe (e.g., bloodstream infection) [1,2]. This organism frequently colonizes the lower respiratory tract of patients with chronic obstructive pulmonary disease (COPD) and is also associated with acute exacerbation of COPD [3]. It is also an important cause of community-acquired pneumonia in adults. In the 1980s, beta-lactamase-negative, ampicillin-resistant (BLNAR) H. influenzae strains were discovered and has increased rapidly in incidence because of overuse of antibiotics, such as cephalosporin [4]. Recently, it the prevalence of BLNAR strains has been reported to increase to nearly 50% in Japan [5,6].
In this report, we described a case of H. influenzae pneumonia that did not respond to sputum culture-guided antibiotic therapy, but was controlled by methylprednisolone.
Case report
A 72-year-old man visited our hospital in May 2016 with complaints of productive cough and mild exertional dyspnea for a week. He had no significant past medical history and current intake of medications, but he was a previous smoker.
Physical examination showed chill, but no shock (temperature, 38⋅7 °C; blood pressure, 120/72 mmHg; heart rate, 86 bpm). His consciousness level was E3V4M5 on the Glasgow Coma Scale, and Coarse crackles and inspiratory rhonchi were heard on the right upper lung field, and his oxygen status was slightly decreased to SpO2 : 95% at room air. 2 of 5 CURB65 severity items were identified. White blood cell (WBC) count was 8,300/μL (neutrophils 72.3%, eosinophils 1.5%, lymphocytes 16.9%, monocytes 8.8%, and basophils 0.5%). C-reactive protein level was 6.69 mg/dL (Figure 1). Chest X-ray identified infiltration shadows in right upper lung field (Figure 2).
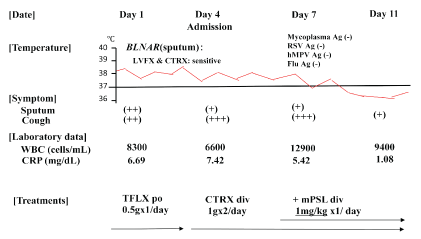
Figure 1: Clinical course of a 72-year-old man with Haemophilus influenzae pneumonia. Administration of TFLX and CTRX was not effective, but administration of mPSL improved his condition. TFLX, tosufloxacin; CTRX, ceftriaxone; mPSL, methylprednisolone
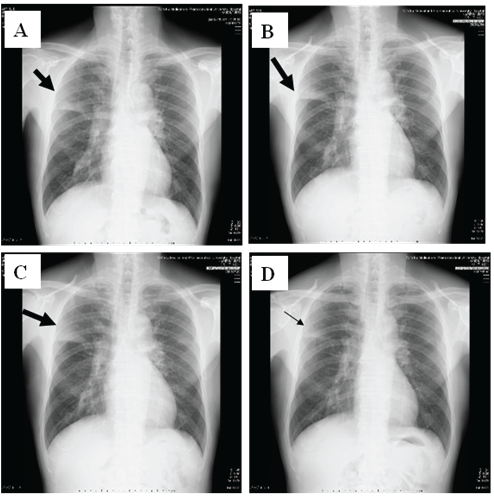
Figure 2: Chest X-ray changes of the patient. A: Day1, B: Day 4, C: Day 7, and D: Day 11, respectively. The infiltration shadows on right upper field did not changed at Day 1 and Day 4, but worsened at Day 7. However, they were improved at Day 11 after methylprednisolone were used.
H. influenzae was isolated from the sputum, with good drug susceptibilities to 3rd generation cephalosporins and fluoroquinolones although our isolated strain was BLNAR which resistant to penicillin (Figure 1). Phagocytosis of the bacilli by many neutrophils (3+) was observed on microscopy and H. influenzae was also semiquantitatively detected at 3+ or >107 cfu/mL. No mycobacterial or Pseudomonas species were collected from the sputum.
Oral administration of Tosufloxacin (TFLX) 0.5g/day was started on Day1 at outpatient clinic, however, his fever and cough did not improve within 48 to 72 hours. 3 of 5 CURB65 severity items were identified. He was admitted our hospital at Day 4 and 1 L/min oxygen was started to be administer due to worsening respiratory status (SpO2 : 94% at room air). H influenzae was not detected from his sputum and blood culture on admission at Day 4. The antibiotic was changed to intravenous ceftriaxone (CTRX), but there was persistence of fever and symptoms, including cough at day 7. The number of WBC in blood was also increased at Day 7. H influenzae and the other bacteria were not detected from his sputum and blood culture at Day 7. Superinfection of some atypical pathogens, such as mycoplasma, respiratory syncytial virus, human metapneumovirus, and influenza virus, was ruled out by negative antigens from his nasal, and pharyngeal swabs.
With suspicions of immunologic mechanisms of pneumonia and/or toxicities of antibiotics that caused non-resolving pneumonia, we added methylprednisolone (mPSL) 1mg/kg/day intravenously from Day 7 (Figure 1). After four days (at Day 11), his symptoms, laboratory data, and chest x-ray findings dramatically improved and we stopped mPSL. The patient was discharged and he kept healthy conditions thereafter.
Discussion
This patient had non-resolving H. influenzae pneumonia despite appropriate antibiotic therapy and general management. Usually, fluoroquinolones and 3rd generation cephalosporins are effective against H. influenzae, which is known as one of the representative pathogens of community-acquired pneumonia. This case showed good drug susceptibility to two major fluoroquinolones: levofloxacin (LVFX) and ciprofloxacin (CPFX) and to a 3rd generation cephalosporin (ceftriaxone), which we used because our isolated strain was BLNAR and resistant to penicillin [6].
We did not specifically investigate the drug susceptibility of the isolated H. influenzae to TFLX, but TFLX belongs to the same family of LVFX and CPFX; hence, it was unlikely that H. influenzae was resistant to only TFLX, one of the oral fluoroquinolones that also covers anaerobes and atypical pathogens, including mycoplasma and chlamydia, but not mycobacterium [7]. Therefore, TFLX were one of the most recommended antibiotics for BLNAR pneumonia cases in outpatient clinic in Japan [8,9]. However, it is important to note that TFLX had been frequently used for otitis media in Japanese children; as a result, emergence of TFLX-resistant bacteria had been a recent cause for concern [7,9]. In fact, the grandchild of this patient received TFLX at the otolaryngology clinic and was found to harbor the same bacteria. We did not know the fact at Day 1, but we should take medical information including his grandsons’ medications more carefully. Our next goal is to investigate TFLX-susceptibility of the isolate from his grandchild.
Bacterial toxicity might be one of the reasons for ineffective use of antibiotics. H. influenzae may be classified as serotypes a to f, depending on the capsular polysaccharide, or as non-typable, if the capsular polysaccharide is undetectable [10]. H. influenzae type b (Hib) is the most common cause of an invasive infection. On the other hand, non-typable H. influenzae (NTHi) is usually the causative organism of mild mucosal infections, such as bronchopneumonia or otitis media, but it has rarely been identified as the causative pathogen of invasive fatal infections.
As the use of Hib conjugate vaccines has become widespread, the incidence of invasive Hib disease has decreased markedly [11,12]. In contrast, the incidence of invasive infection caused by NTHi has increased; this phenomenon is known as pathogen shift [2]. In our case, the isolated strain was NTHi. In addition, characteristics of H. influenzae other than the particular capsular polysaccharide, or its absence, might explain the absence of response to adequate antibiotic therapy in this case. Further studies are needed to validate these hypotheses.
Some immunologic mechanism might have been present in our antibiotic-refractory case. Previously, we reported a case of invasive NTHi respiratory tract infection with a large quantity of neutrophil extracellular traps (NETs) in sputum [2]. NETs are fibrous structures that are released extracellularly from activated neutrophils during inflammation, such as in pneumonia, and rapidly trap and kill pathogens as a first line of immunologic defense [13-16]. However, the function and pathologic roles of NETs have not been fully investigated, and recently, excessive immunologic function was suggested to cause tissue damage when NETs showed extremely amount and extend as ‘double-edged sword’ [17-19]. Our patient had no history and backgrounds of atopy and immunologic diseases, but mPSL dramatically decreased inflammation, chest X-ray infiltrations, and symptom, including cough. The presence of NETs and excessive neutrophil reaction might have enhanced his severe pneumonia. We unfortunately did not perform bronchoscope to diagnose the other nonresolving pneumonia, including organizing pneumonia (OP) because the patient refused further examinations. Previously, it was reported that H influenzae were isolated 3 of 10 OP with aggregates neutrophils patients, but none of other 4 OP without neutrophils infiltration cases [20]. These results suggested the relationship OP with neutrophils and H influenzae. Further analysis about the mechanisms of steroid effectiveness in these cases should be needed.
In steroid use, Torres A et al examined acute use of mPSL 0.5mg/kg BID for 5 days (n=61), and found less treatment failure compare with placebo (n=59) in community-acquired pneumonia suggested with high inflammatory status [21]. The use of corticosteroids is not routine clinical practice and there are no official recommendations yet, but we should investigated additional use of mPSL in severe and/or unresolving pneumonia cases, including our presented patient.
Conclusion
In conclusion, we presented a case of H. influenzae pneumonia that did not improve despite culture-guided antibiotic therapy. Although the possibility of TFLX resistance cannot be ruled out, some other immunologic and toxic mechanisms, such as the presence of NETs, may have also contributed. Further investigation on the pathogenicity and immunopathology of H. influenzae infection in this era of widespread Hib vaccine use should be pursued.
Conflict of Interest
None
Acknowledgement
This work was supported by the Japanese Society for the Promotion of Science Grant-in-Aid for Scientific Research 26461158 (to M.S.).
Article Information
Article Type: Case Report
Citation: Seki M, Oikawa N, Hariu M, Watanabe Y (2017) Methylprednisolone for Antibiotic-Refractory Haemophilus influenza Infection. J Infect Pulm Dis 3(1): doi http://dx.doi.org/10.16966/2470-3176.121
Copyright: © 2017 Seki M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 07 Jan 2017
Accepted date: 05 Feb 2017
Published date: 10 Feb 2017



