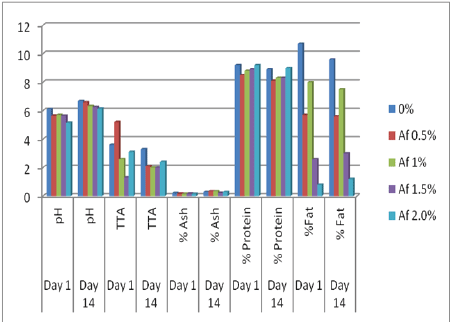
Figure 1: The effect of different concentrations of A. danielli (Af) on the acidity, pH, protein, fat and ash content of peanut milk on day 1 and day 14


Gabriel O Adegoke1* Adediwura T Falade2
1Department of Food Technology, University of Ibadan, Ibadan, Nigeria*Corresponding author: Gabriel O Adegoke, Department of Food Technology, University of Ibadan, Ibadan, Nigeria, E-mail: goadegoke@yahoo.com
Peanut milk is a type of imitation milk derived from peanuts and it is known to be lactose-free and nutritious therefore it is suitable for lactose-intolerance people. The aim of the study is to produce peanut milk, use Aframonum danielli (A. danielli) as natural preservative and Carboxyl methylcellulose (CMC) as a stabilizer. Also to determine the effect of A. danielli at different concentrations on the physical, chemical and microbial profiles of peanut milk stored for two weeks. This study is necessary because there are no reports in literature on the effects of A. danielli and CMC on the physical, chemical and microbiological characteristics of peanut milk. Peanut milk was produced from peanuts and flavored with A. danielli extract at different concentrations (0.5%, 1.0%, 1.5% and 2.0%). pH, titratable acidity, protein, fats, ash, moisture content, total viable count and coliform count were determined. A storage study was carried out for two weeks at room temperature and refrigeration temperature. Sensory evaluation was conducted using the 9 point hedonic scale of which 9 represents like extremely and 1 represents dislike extremely. There was a general decrease in the pH of the peanut milk samples as compared to the control sample on day 1 and an increase on day 14. On day 1, there was a general decrease in the % fat content of all the samples when compared with the control sample. The sample with 2% A. danielli had the highest fat reduction rate. On day 14, there was further decrease in the % fat content of all the samples except the 1.5% and 2% A. danielli samples which showed a slight increase. However, the 2% sample with A. danielli still had the lowest % fat content. The pattern of decrease in the % protein content on day 1 and day 14 were similar. The % moisture content of all the samples varied for day 1 and day 14. The % ash content of all the samples decreased on day 1 and increased by day 14. On day 14, the control sample had the highest total viable counts. Also on day 14, all the samples had no coliforms. The sensory evaluation test revealed that, the peanut milk sample containing 2% A. danielli had the most acceptable sensory parameters.
Peanut milk; A. danielli; CMC; Lactose-free
Peanut (Arachis hypogaea) is a herbaceous, annual legume, originating in South America. The pods of the peanut develop in the ground and hold one or more kernels that contain 38-50% oil and are also rich in protein. It produces its flower above the ground and produces its pod underground hence its name groundnuts. Due to its edible oil and protein meal content, it is therefore considered to be highly valuable in human and animal nutrition [1]. Though groundnuts are nutritious and can be effective in reducing malnutrition, the consumption rate in developing countries is low and therefore should be encouraged. This can be done by developing new products from groundnuts that will attract consumers [2]. This may include its use as imitation milk such as peanut milk.
Imitation milk has been used to describe products that have the characteristics of milk but do not have any form of milk fat nor dairy ingredient [3]. An imitation milk derived from a plant source can be said to be plant milk. Peanut milk is imitation milk derived from peanuts (groundnuts). Peanut milk can also be said to be a non-dairy beverage created using peanuts, water and (depending on the recipe) possibly other ingredients like sea salt and sweetener. It does not contain any lactose and is therefore suitable for people with lactose intolerance. The method used for the production of almond milk, soy milk and rice milk can also be applied in the production of peanut milk. This involves grinding, soaking, sometimes heating, and then filtering through a muslin cloth. The filtrate is regarded as the ‘milk’. Soybean milk or peanut milk can be used to improve the diets of pre-school and school children [4]. It can also be used in place of ordinary milk in the case of lactose intolerance [4]. In spite of successes recorded so far, more work still needs to be done to improve the stability, reduce or completely eliminate the nutty flavor, and sensory problems [5], which are the major challenges experienced when producing peanut milk and some peanut milk based products [5].
According to Howell [6], the use of natural occurring materials as preservatives is a promising alternative to the use of chemicals. The potential sources of natural preservative are spices, herbs, fruits, seed, leaves, barks and roots. The strong association between increased consumption of these natural products and human diseases prevention has been explained by the content of the phytonutrients [7]. Spices are common food adjuncts that impart flavor, aroma and color to foods. Several common spices are now understood to exert many beneficial physiological effects [8]. Among these, their hypolipidemic and antioxidant properties have far-reaching health implications.
A. danielli (oburo) is a spice belonging to the family Zingiberaceae. The fruits are initially green and later become reddish-brown when ripened. It is a spice grown in the tropical zone in Nigeria, it is mostly found growing in the riverine or forest zone of Delta, Edo, Rivers, Lagos, Imo, Cross river and Ondo state. This spice has seeds that are used in a very small quantity in ground form as flavoring agent for traditional dishes and pepper-soups. The essential oil is used for perfumery and dye preparations. It is known to possess preservative properties having both antibacterial and antifungal effects [9,10]. Adegoke et al. [11] reported that A. danielli has been successfully used to preserve maize and soybean against mould growth and insect infestation for 15 months without adversely affecting their growth rate. It has a potent synergistic inhibitory effect on food spoilage yeast when used in combination with hydrostatic pressure [12]. It has also been found to inhibit the growth of some foodborne pathogens like Aspergillius flavus, Aspergillus parasiticus and Aspergillius ochraceus [10]. A. danielli contains varying amounts of minerals like calcium, magnesium, sodium, manganese, phosphorus, zinc and copper, as well as a number of amino acids like L-Threonine, L-Serine, L-Valine, L-Proline, L-Glutamic acid, glycine, L-Leucine and L-Lysine [10]. Adegoke and Gopala Krishna [13] reported that the spice contains antioxidant component of phenolic compounds with hydroxyl grouping, having reducing properties which may delay or inhibit auto-oxidation. Fasoyiro et al. [14], while investigating the anti-oxidant property of A. danieli extract in oil compared its activity with other antioxidants of plant sources and concluded that A. danielli was more effective than tocopherol in reducing peroxidation in soybean oil.
Carboxyl methylcellulose (CMC) is a polysaccharide which is widely used in many industrial sectors including food, textiles, paper, adhesives, paints, pharmaceutics, cosmetics and mineral processing. It is a natural organic polymer that is non-toxic and biodegradable. These properties make it ideal for industrial applications. It is used as a viscosity modifier or thickener and to stabilize emulsions in various products [15].
The objectives of the study are to produce peanut milk and also determine the effect of A. danielli on the physicochemical, microbial, sensory and storage parameters of the peanut milk.
A. danielli seeds were purchased from Bodija market in Ibadan, Oyo State, Nigeria in November, 2010. The seeds were sundried and dry milled using a blender. The resulting powdery substance was weighed as required (0.5 g in 100 ml of distilled water, 1.0 g in 100 ml of distilled water, 1.5 g in 100 ml of distilled water and 2.0g in 100 ml of distilled water) into pet bottles. The PET bottles were put into a polythene bag and left for 4 days in a dark cupboard after which filtration was done using filter paper. The resulting extract was kept at room temperature until required for use. Carboxyl methylcellulose (CMC) powder was purchased from Bodija market in Ibadan, Oyo State, Nigeria. Half a gram of CMC powder was weighed into 100 ml of distilled water and kept at room temperature until required for use. The method described by Ojofeitimi et al. [16] was used for the preparation of the peanut milk with some modification to suit the present study. Peanuts (bororo) obtained from Bodija market, Ibadan Oyo State, were soaked in water at room temperature for about 8 hours and cooking was done for about 15-20 minutes on high heat after which blending and sieving using a muslin cloth was carried out. The milk-like extract was cooked again for about 10-15 minutes (on high heat) or until it started to boil. The amount of sugar syrup added depended on the rate of sweetening desired. The sweetened peanut milk was divided into six portions of which one portion served as the control (no additive was added). Five milliliters of CMC and 25 ml of A. danielli extract were added to the remaining portion at the required concentrations.
pH: The pH of the samples were determined using standard method [17]. The pH meter was standardized using buffer 4,7 and 9 solutions. The electrode was rinsed properly with distilled water to establish equilibrium. The sample was poured into a 100ml beaker and the pH was taken.
Titratable acidity: The titratable acidity of the samples was determined using standard method [17]. 10 ml of the peanut milk was measured into a conical flask using a pipette. Two drops of phenolphthalein indicator was added. The solution was titrated against 0.1M standard of sodium hydroxide (NaOH) solution until color change was noticed and titre values were recorded.
Moisture content: The moisture content of the samples were determined using standard method [17]. A clean flat dish made of silica, was dried in an oven for one hour. The flat dish was transferred into a dessicator to cool. The cooled flat empty dish was weighed and the weight recorded as (w1). About 2 grams of the test substance was spread into the dish and weighed accurately. The weight was recorded as (w2). The dish and its contents were transferred into the desiccator to cool. The new weight of the flat dish and its dried contents was accurately taken on a balance. The dish was returned into the air oven for 1hour and cooled in the desiccator and weighed accurately. The weight was recorded as (w3).
Calculation\[\frac{{(w2 - w3) \times 100}}{{(w2 - w1)}}\]
Ash content: Percentage ash content was determined as determined as described by AOAC [17]. The silica dish was weighed and recorded as w1. Two to five grams of the test sample was weighed into the dish and the weight was recorded as w2. The test substance was evaporated to dryness on a boiling water bath. The dried sample was charred over a Bunsen flame in a fume cupboard until no more soot was given off. The dish was transferred using a pair of tongs into a muffle furnace set at 550°C. Ashing was completed when the charred sample was completely white in colour. With the aid of a pair of tongs the dish and its content was transferred into the desiccator to cool. The dish and its content was weighed and recorded as w3.
Calculation: [17]\[\frac{{(w3 - w1) \times 100}}{{(w2 - w1)}}\]
Fat content: The Mac-cartney bottles were weighed empty (a grams). 3 grams of sample was weighed into the Mac-cartney bottles (b grams). Petroleum ether was added to the weighed sample in the weighed Maccartney bottles and shaken well to facilitate fat removal. The fat in solvent was collected into another weighed Mac-Cartney bottle. Evaporation of the solvent was done by placing Mac-cartney bottle containing the fat in solvent in an oven. When evaporation was completed, the bottle now containing fat was weighed as (d grams) and the percentage fat was calculated as follows:
Calculation:
\[\frac{{(d - {\rm{c}}) \times 100}}{{(b - {\rm{a}})}}\]
Protein content: Three grams of the sample was weighed into the kjeldahl flask. Concentrated sulphuric acid was added and the mixture became black. The mixture was heated until the black material became a colourless viscous liquid. The liquid was then transferred into a distillation set where concentrated NaOH was added to neutralize the concentrated acid and ammonia was released. Ammonia was collected into an acid solution (Boric acid with an indicator).This was back titrated against acid.
\[\frac{{0.014 \times {\rm{titre}} \times 6.25 \times 100}}{{{\rm{weight}}\,{\rm{of}}\,{\rm{sample}}}}\]
The methods described by AOAC [17] were used for total viable and coliform count. The total viable and coliform counts were carried out using plate count agar (PCA) and eosin methylene blue agar (EMB) respectively. All materials where sterilized before use. Nine ml of sterile peptone water was dispensed into the test tubes under the lamina flow and labeled 1-10. The stock sample was homogenized and 1ml of the peanut milk with different concentrations of A. danielli was pipetted into 9 ml of peptone water to give 10-1dilution and mixed very well using a vortex. From the 1st dilution 1ml was pipetted into the next tube to give 10-2 and this was continuously done until the tenth tube. 0.1ml of the desired dilution was dispensed into sterile petri plate and 20 ml of the already sterilized and cooled PCA/EMB agar was poured into the plate. Swirling was done clockwise, anticlockwise, forth and back to ensure proper mixing of the bacterial cell. The sample was allowed to solidify and the plates were inverted in the incubator that was set at 37°C. Growth was observed after 24hrs and cells were counted.
The peanut milk samples were stored at room temperature and refrigeration temperature for two weeks after which samples were analyzed for taste, aroma and color changes using human senses.
Sensory evaluation was carried out using the 9 point hedonic scale where 9 represents like extremely and 1 represents dislike extremely and the results were analyzed using multiple comparison test.
According to figure 1, there was a general decrease in the pH of the samples as compared to the control sample on day 1 and an increase in pH of all the samples on day 14. The titratable acidity values of 2.0%, 1.5% and 1.0% concentrations of A. danielli flavored samples exhibited a decrease on day 1 while 0.5% A. danielli flavored samples exhibited an increase in the titratable acidity value as compared to the titratable acidity of the control sample on day 1. On day 14, all the titratable acidity values of all treated samples except the 0.5% A. danielli sample decreased while the titratable acidity of the control sample remained high. There was a general decrease in the % fat contents of all the samples on day 1. On day 14, there were further decreases in the % fat contents of all the samples except the 1.5% and 2% A. danielli samples which showed a slight increase. The 2% A. danielli had the highest fat reduction rate on day 1 and day 14. The patterns of decrease in the % protein content of the A. danielli flavored samples were similar for day 1 and day 14. It was observed that the higher the concentration of the A. danielli in the samples the lower the rate of decrease in the % protein content of the sample. The % moisture content of all the samples varied for day 1 and day 14. The % ash content of all the samples decreased on day 1 and increased by day 14.

Figure 1: The effect of different concentrations of A. danielli (Af) on the acidity, pH, protein, fat and ash content of peanut milk on day 1 and day 14
With reference to table 1, all the samples contained a certain number of total viable and coliform count on day 1. On day 14, the control sample had the highest total viable counts while all the samples had no coliform count. After a week of storage at room temperature (26 ± 2°C) all the samples investigated had separated into three layers (a chalky layer, clustered layer and watery layer). At the end of the second week, further separation was observed. Samples stored at refrigeration temperature after about 24 hours exhibited increase in viscosity and showed no sign or separation after a week. However at the end of the second week, clogging was observed but separation was not evident. The samples had a slight putrid odor and color change. General acceptability difference between all the peanut milk samples investigated was not significant at 5% and 1% levels (Table 2).
| Treatments | Total viable count (cfu/ml) Day 1 | Total viable count (cfu/ml) Day 14 | Coliform count (cfu/ml) Day 1 | Coliform count(cfu/ml) Day 14 |
| Peanut milk with no A. danielli |
1.0 × 109 | TNTC | 4.4 × 108 | NG |
| Peanut milk with 0.5% A. danielli | 2.3 × 109 | NG | 1.2 × 109 | NG |
| Peanut milk with 1% A. danielli | 1.8 × 109 | 1.0 × 108 | 7.9 × 108 | NG |
| Peanut milk with 1.5% A. danielli | TNTC | 1.0 × 108 | 1.6 × 109 | NG |
| Peanut milk with 2.0% A. danielli | 1.2 × 109 | 6.0 × 107 | 4.4 × 108 | NG |
Table 1: The effect of different concentrations of A. danielli on the total viable count and coliform count of peanut milk on day 1 and day 14
TNTC: too numerous to count NG: no growth
| Analysis of variance table for overall acceptability | ||||
| Source of variation | df | ss | ms | f |
| Samples | 5 | 5.35 | 1.07 | 0.58 |
| Judges | 9 | 58.02 | 6.45 | 3.52 |
| Error | 45 | 82.48 | 1.83 | |
| Total | 59 | 145.85 | ||
Table 2: Sensory evaluation analysis of variance table for overall acceptability of peanut milk with different concentratio ns of A. danielli
It can be deduced from the result that the A. danielli extract brought about a reduction in the pH of the sample on day 1 probably due to the amount of acid in the extracts or their microorganisms. The increase in pH on day 14 may be due to the storage temperature or the duration of storage. This correlates with the findings of Ashaye et al. [18], when they worked with the effect of local preservatives (A danielli) on the chemical and sensory properties of stored warakanshi. Ashaye et al. reported that at cold temperature especially on the 6th day of storage, slight increase in pH was detected [18]. This increase in the pH value can also be explained by Willey et al. [19], that microorganisms frequently change the pH of their own habitat by producing acidic or basic metabolic waste product. The control sample exhibited the highest rate of decrease on day 14 probably due to a higher rate of fat oxidation in the control sample as a result of the absence of A. danielli extract which have been reported to have antioxidant properties [14]. The pattern of decrease in the % protein content of the A. danielli flavored samples on day 1 and day 14 suggested that the higher the concentration of the A. danielli used in the sample, the lower the rate at which the protein can be used up probably by microorganisms. According to Potter and Hotchkiss [3], an organism can be inhibited from further growth by its own acidity, so it is possible that at 2% A. danielli concentration, it can be said that the antimicrobial property of the A. danielli was sufficient to inhibit the activity of its microorganism. Fluctuations observed in the values of the % moisture content of the samples can also be explained using the findings of Ashaye et al. [18], on the effect of local preservatives (A. danielli) on the chemical and sensory properties of stored warakanshi. The authors suggested that fluctuations in moisture content were probably due to the activity of microorganisms and catabolic enzymes produced by them. The decrease in % ash content of all the samples on day 1 may be as a result of the microorganisms present in the extracts using up the minerals in the peanut milk sample thereby bringing about a reduction in its % ash content. The increase in % ash content on day 14 may be due to the minerals produced as a result of the death of microorganisms initially present in the sample (this include the microorganism in the extract and the peanut milk itself).
The control sample having the highest total viable counts on day 1 may be due to the absence of the A. danielli extracts acting as natural preservatives. This correlates with the findings of Adegoke et al. [9] and Adegoke and Skura [10], that A. danielli is known to possess preservative properties having both antibacterial and antifungal effects. The absence of coliforms in all the samples on day 14 may be due to the presence of other microorganisms in the peanut milk making the environment unfavorable for the growth of the coliform cells over the period of storage.
The separation observed in the peanut milk after one week and at the end of the second week of storage suggest that the stabilizer (CMC) used was either not effective or the concentration used was not sufficient enough to bring about stabilization. Potter and Hotchkiss [3], noted that yeast would tolerate moderately high acidic conditions and produce alkaline end products such as ammonia from the breakdown of protein thereby neutralizing previously formed acid and permit subsequent growth of proteolytic and lipolytic bacteria. Also, Willey et al. [19] explained that when foods contain large amounts of proteins and or fats, bacterial growth can produce a variety of foul odors. The anaerobic breakdown of proteins yields foul smelling amine compounds, a process called putrefaction. One of the major sources of odor associated with putrefaction is organic amine cardaverine. Therefore, the putrid odor of peanut milk samples stored at room temperature was probably due to the activity of spoilage organisms which may be some kind of bacteria or yeasts that the A. danielli extracts was not effective against probably at the concentrations used.
All the sensory attributes of the samples treated with different levels A. danielli showed no significant difference at both 1 % and 5 % levels.
At 2% concentration, the lowest pH value was obtained for day 1 and day 14 indicating that it probably has the highest preservative property since preservative properties is more favored at a lower pH. The titratable acidity of the 2% concentration of A. danielli, though not the highest value at day 1, had the highest value (amongst the other concentration of A. danielli used) by day 14 also indicating a higher preservative property. The % protein content of the sample containing 2% A. danielli on day 1 and day 14 was the highest compared to the other concentrations used. The 2% concentration also had the highest effect with respect to fat reduction in the peanut milk sample. Based on the concentrations used and the parameters measured in this study, adding A. danielli at a concentration of 2% to peanut milk has the most desirable effect on the physicochemical, microbial and sensory characteristics of peanut milk. The most suitable means of storage of peanut milk can be concluded to be cold storage (refrigeration temperature) because the rate of deterioration will be slower than will occur at room temperature. However, the sample should be consumed in less than a week even when stored at refrigeration temperature.
Download Provisional PDF Here
Article Type: Research Article
Citation: Adegoke GO, Falade AT (2016) Effect of Aframomum danielli on Peanut Milk Characteristics and Flavor. Nutr Food Technol Open Access 3(1): doi http://dx.doi.org/10.16966/2470-6086.136
Copyright: © Adegoke GO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access