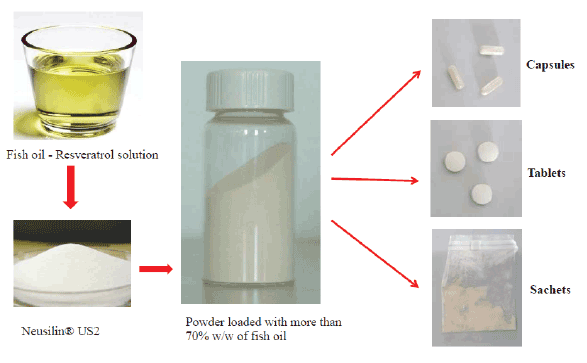
Figure 1: A versatile and stable mixture of fish oil and resveratrol in a powder formulation

Mosè Santaniello Giuseppe Giannini*
Department of Research and Development, Sigma-Tau Industrie Farmaceutiche Riunite S.p.A, Pomezia, Roma, Italy*Corresponding author: Giuseppe Giannini, Department of Research and Development, Sigma- Tau Industrie Farmaceutiche Riunite S.p.A, Pomezia I-00071, Roma, Italy, Tel: +39.0691393640; E-mail: giuseppe.giannini@sigma-tau.it, mosè.santaniello@sigma-tau.it
Highly unsaturated n-3 PUFA (polyunsaturated fatty acids) and resveratrol are known to be two prominent healthy components of modern diet. Fish oil is the main source of n-3 PUFA, which plays an important role in the prevention of different human diseases. However, fish oil is highly prone to oxidation, leading to the production of off-odors and off-flavors. Thus, natural and synthetic antioxidants are most commonly used. We have investigated a number of antioxidant agents. Resveratrol, a natural antioxidant found in the skin of red grapes, as well as in other sources such as peanuts and berries was the most effective in stabilizing solutions of fish oil absorbed on an inert support. It is an excellent ingredient with broad-spectrum prevention linked to cardiovascular disease, cancer, diabetes and Alzheimer’s disease, but also for anti-aging benefits. Mixtures containing fish oil and resveratrol are widely reported in literature as liquid or microencapsulated formulations. When the fish oil, as all polyunsaturated fats, is absorbed on a porous solid support, there is a huge increase of the surface, for which the oxidation process is greatly accelerated facilitating also the self-oxidation (autoxidation). Here we present a powder formulation, containing a bioactive mixture of fish oil and resveratrol, with apparent excellent rheological properties and stable for at least 6 months (>96%) when stored at 25°C. A useful tool in daily nutritional supplementation, focused to prevent illness and disease and to improve general well-being.
Fish oil; n-3 PUFA; Resveratrol; Antioxidant
n-3 PUFA: Omega-3 Polyunsaturated Fatty Acid; DHA: Docosahexaenoic Acid; EPA: Eicosapentaenoic Acid; HPLC: High- Performance Liquid Chromatography; RH: Relative Humidity
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are two n-3 polyunsaturated fatty acids found mainly in fish oil and fishmeal; both of them play a vital role in human health and nutrition and are of great importance in the food and pharmaceutical industries. Well known are the multiple health benefits of fish oil supplements that include a lower risk for heart attack and stroke in those with cardiovascular disease, associated with lowering blood pressure and triglycerides as well as slow plaque buildup in the arteries. Recent findings have also highlighted the role of omega-3 fatty acids in the modulation of the myogenic program of the stem cell population within skeletal muscles [1]. That is why fortification of foods with such fatty acids is increasingly recommended. Resveratrol is a natural antioxidant agent, isolated from grapes and other food products, described for years as endowed with remarkable health properties. Yet there is still not a unique position on the effectiveness of resveratrol in the international scientific community [2]. The fish oil-resveratrol association was identified as effective to be stable when absorbed on an inert support in order to make a powder formulation [3,4]. We report the results of a study where various antioxidants have been investigated and even a couple of inert support among those accepted for human use. A study of stability at different temperatures and an accelerated stability have allowed identifying the right combination of fish oil/antioxidant/inert support, stable for at least six months at room temperature.
n-3 PUFAs are a family of essential fatty acids with many biological activities. A family of long-chain polyunsaturated fatty acids, generally C16-C24, in particular those having a C18-C22 chain represent the three major dietary n-3 fatty acids, α-linolenic acid (ALA; 18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3), and docosahexaenoic acid (DHA; 22:6n-3). They all have in common a carbon-carbon double bond in the n-3 position. Omega-3 fatty acids comprise cell membrane phospholipids, contributing to structural and functional characteristics by altering signalling platforms for membrane proteins and lipids. It has been shown beneficial effect in the prevention of cardiovascular events [5], possibly by means of an anti-inflammatory, antithrombotic and antiarrhythmic mechanism. The hypolipidic effect was the first detected, so at first these drugs had been used for the treatment of dislipidemic disorders, while the anti-inflammatory, antithrombotic, anti-atherosclerotic and antiarrhythmogenic effects have been found later [6]. GISSI-Prevention trial [7] was the first trial demonstrating the efficacy and tolerability of n-3 PUFAs in post-myocardial infarction patients. According to the evidence in literature, today n-3 PUFAs are indicated for the primary and secondary prevention of ischemic cardiopathy and sudden cardiac death (SCD) [8,9]. Recently, n-3 PUFA has been claimed even as essential for neural development and function [10]. Taken together, these data suggest that n-3 PUFA are fundamental component of human diets, and fish oil is an important source.
Fish oil is produced by different methods such as wet pressing, cold extraction, wet reduction or enzymatic extraction, supercritical fluid extraction, etc. [11-14]. In all cases, the product obtained is a liquid easily oxidizable due to the high degree of unsaturation of its ingredients. Industrially, fish oil is often marketed as natural grade in soft gel capsules and oil-filled hard capsules, with the advantage for the consumer to be without fishy smell; rarely as syrup because of its bad taste. Fish oil can also be used as a vehicle for other pharmaceutically active ingredients such as ketoprofen [15], statins [16], etc. [17], and a solid oil formulation resulted in higher patient compliance towards prevention therapy. Interested readers are encouraged to consult recent reviews to get a broader understanding of this area [18-20]. Many patents claimed formulation of fish oils in solid pharmaceutical, dietetic or alimentary compositions, but it is still industrially underdeveloped.
Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a natural stilbene, and aphytoalexin, produced naturally by several plants in response to injury or when the plant is under attack by pathogenic infections such as bacteria or fungi [21]. Resveratrol is isolated from grapes and other food products such as peanuts, bilberries, blueberries, cranberries, raspberries, and mulberries, with antioxidant property and potential chemopreventive activities. It is known that it has cardioprotective effects, and different mechanisms of action have been proposed [22,23]. However, there is still not a unique position on the effectiveness of resveratrol in the international scientific community. Indeed, in 2014 a meta-analysis found that resveratrol supplementation at usual doses has no effect on blood pressure [2], while its efficacy on cancer in humans is inconsistent [24]. In a patent, EP1567137B1, it describes the use of resveratrol for treating influenza virus infections [25]. It also acts as antioxidant and skin protecting agent [26]. So, as antioxidant is still widely used worldwide as a dietary supplement.
Fish oil is highly prone to oxidation, leading to the production of off-odors and off-flavors. Thus, natural and synthetic antioxidants, like resveratrol, are most commonly used. Mixtures containing fish oil and resveratrol are widely reported in literature as liquid or microencapsulated formulations. A further strategy to increase the stability of oily substances of pharmaceutical or nutritional interest is to transform them into solid dispersions. This operation can be carried out through the microencapsulation [27] or through the preparations of systems “solidliquid” [28]. The micro-encapsulation consists in covering micro droplets of emulsified oil with a solid matrix generally through spray-dryer, while the realization of solid-liquid systems, or more generally of the solid dispersions is effected by adsorbing on inert supports of inorganic nature, the oily phase (oils or oily solutions) which retains its physical state, finally achieving a solid usable for common oral formulations. However, it is rare to find fish oil absorbed on the solid matrix on the market. Numerous studies have been published in an effort to propose solid formulations of fish oil, primarily microencapsulation by the spray drying method, often using the same resveratrol as antioxidant agent [29,30]. However, there are only few papers concerning oil absorbed on the solid matrix, and some of them are patents [31].
Here we report the results of a study aimed at obtaining fish oil in powder form, with apparent excellent rheological properties and are stable for at least 6 months at room temperature. To this purpose, it was necessary to minimize the exposure to oxygen and use inert substrates to adsorb fish oil.
Oxygen removal (packaging) or oxygen scavengers (formulation), as in the case of a powder formulation, are the antioxidant strategies commonly employed to prevent or delay fish oil oxidation. A number of natural antioxidants such as ascorbic acid and ascorbylpalmitate, resveratrol, α-tocopherol, coenzyme Q10, epigallocatechin, hydroxytyrosol, oleorupine, and lipoic acidwere investigated as oxygen scavengers. Among all of them, resveratrol has shown to be a very good antioxidant for fish oil.
Regarding the inert substrates where to absorb the fish oil solution, forming a readily handle able powder, two versatile excipient commonly used for drug formulations, Neusilin® US2 and Syloid® XDP, were explored.
In order to have fish oil as solid formulations, so far many systems have been explored, and patented. However, few are commonly used while the most widely formulations used are capsules: soft and hard gelatin and microencapsulation. Capsules showed a number of advantages: protection of fish oil from exposure to oxygen and other ingredients, can be filled only with fish oil or with oil-based mixtures, and are easy to take. The microencapsulation have also the advantage that they can be mixed with other ingredients/powders “external” to the microcapsule and be formulated in sachets or tablets, as for all the powders. However, the industrial technology for the production of capsules is always more complex than for the formulation of powders. Therefore, several studies have been performed in order to absorb the fish oil on inert solid matrix, and convert oil into stabilized fine powders. Often, however, these technologies showed two limits, the amount of oil adsorbed was not sufficient enough to achieve the therapeutic dosage and stability of the powders was not compatible with an industrial production.
Several studies have been performed in order to absorb the fish oil on inert solid matrix, and convert oil into stabilized fine powders. A number of natural products were investigated as antioxidant agents (Table 1). Among these, resveratrol has ensured greater fish oil stability versus other agents investigated. As inert substrate was selected a fine ultra-light granule of magnesium aluminometasilicate (Neusilin® US2; chemical Formula Al2O3- MgO-1.7SiO2.xH2O, CAS Number 12511-31-08). Due to its large surface area and porous nature, Neusilin® US2 can to adsorb high loads of oil and to be mechanically compacted into high quality tablets.
A second inert substrate, the Syloid® XDP silicas, was also investigated. They are mesoporous, amorphous, silica gel excipients, usually used to create an excellent solid porous carrier for pharmaceutical formulations.
Such fish oil formulation can be useful for the preparation of pills, tablets, capsules or sachets, as well or in combination with other active ingredients (Figure 1).

Figure 1: A versatile and stable mixture of fish oil and resveratrol in a powder formulation
Stability test of this formulation was performed under normal or accelerated conditions, at 25 and 30°C at 60% RH (relative humidity), and at high temperature (40°C; at 70% RH), up to six months. The recovery of n-3 PUFA is at least 96% after 6 months at 25°C at 60% RH; at least 95% after 3 months at 30°C at 60% RH; and at least 90% after 3 months at 40°C at 70% RH.
For the purpose of the present study, the following materials have been purchased:
a) A mixture of ethyl esters of n-3 PUFA, with a content in EPA and DHA greater than 85%, in a ratio EPA/DHA comprised between 0.9 and 1.5, (Pronova, Norway); b) Resveratrol (Royalmount Pharma, Montreal, Canada); c) Neusilin® US-2 (Fuji Chemical Industry Co., Ltd, Japan); d) α-tocopherol (Sigma-Aldrich s.r.l., Milan, Italy); e) Syloid XDP (Grace Performance Chemicals, Worms, Germany); f) Coenzyme Q10 (Sigma- Aldrich s.r.l., Milan, Italy); g) Ascorbylpalmitate (Sigma-Aldrich s.r.l., Milan, Italy); h) Lipoic acid (Aldrich s.r.l., Milan, Italy); i) Ascorbic acid (Sigma-Aldrich s.r.l., Milan, Italy); j) Hydroxytyrosol (Probeltebio Murcia, Spain); k) Epigallocatechin (Sigma-Aldrich s.r.l., Milan, Italy)
A general method for the preparation of formulations involved the addition of the antioxidant agent to fish oil (n-3 PUFA); the solution was left under mechanical stirring at 600 rpm for 3 hours at 22°C at 60% RH; a clear solution was observed. It was then added to inert solid support (Neusilin® US2 or Syloid® XDP) in small portions, manually mixing up to obtain a homogeneous solid.
The formulations stability was evaluated under normal or accelerated conditions, at 25 and 30°C at 60% RH (relative humidity), and at high temperature (40°C; at 70% RH), up to six months.
The HPLC analysis was performed using a Column Symmetry C-18 4.6 × 150 mm, a solution of CH3CN/CH3OH/H2O 45/45/10 as eluent, a flow of 1 ml/min and a Mass Spectrometry Detector.
Stability analysis under accelerated conditions was performed by radiation and by high temperature according to the following procedure:
A number of formulations of fish oil (n-3 PUFA) absorbed on Neusilin® US2 were prepared; various antioxidants were investigated and the results compared to the counterparty without antioxidants (Table 1).
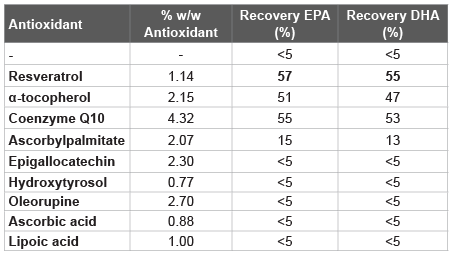
Table 1: Accelerated stability testing (by radiation; at 22°C for 24 h) with Formulation 1 and with antioxidant agents
Formulation 1 (the counterparty without antioxidants): n-3 PUFA (5.0 g) was added to Neusilin® US2 (5.0 g) in small portions, manually mixing up to obtain a homogeneous solid. Stability tests were carried out at 25°C at 60% RH, 30°C at 60% RH and 40°C at 70% RH as described above for 1, 3 and 6 months (Table 2). After three months at 40°C and after 6 months at 25°C, all DHA and EPA were completely degraded.
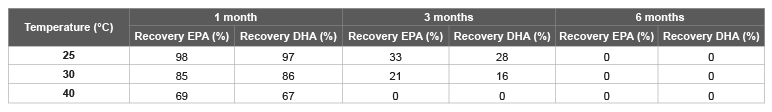
Table 2: Stability test with Formulation 1
From here, a number of formulations were prepared by adding various natural antioxidant agents to Formulation 1, and evaluated for accelerated stability testing after autoxidation induced by radiation, in order to assess the inhibition of the degradation of omega-3 DHA and EPA (Table 1). Furthermore, the same solutions were evaluated after 30 days at 60°C (Table 3).
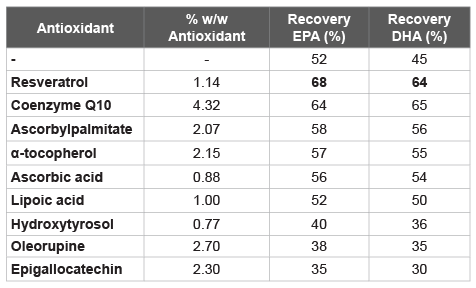
Table 3: Stability test with Formulation 1 and with antioxidant agents, at 60°C for 30 days
In both preliminary stability tests reported above, resveratrol, as well as Coenzyme Q10 and α-tocopherol, showed a better performance. Therefore, other formulations were prepared using these three antioxidant agents (Table 4). Each formulation contain 1.0% of antioxidant agent while for Formulation 5 was added 12% of resveratrol. A stability test was performed at three temperatures, 25, 30, and 40°C for 3 months (Table 5).
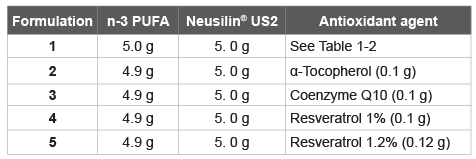
Table 4: n-3 PUFA/Neusilin-based Formulation 1 with three selected antioxidant agents
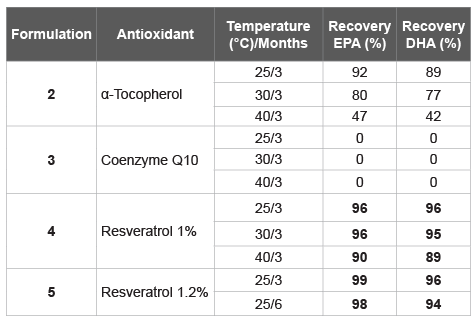
Table 5: Stability test with three selected antioxidant agents, at three temperature, 25, 30, and 40°C, for 3 months
In the case of resveratrol, the most efficient antioxidant among those tested, a slight increase in the percentage-from 1.0% (Formulation 4) to 1.2% (Formulation 5)-has allowed a further increase in stability. Formulation 5 was the best among those investigated in this study.
Aside from Neusilin® US2, other inert solid support is still available. Here we have also tested Syloid® XDP (Table 6), which has proved less efficient than Neusilin® US2 (Tables 7). In detail, two Syloid® XDP-based formulation of fish oil were investigated, without (Formulation 6) and with an antioxidant agent (Formulation 7), the latter analogue to Formulation 4. Both of them, at 25°C for 24 h as well as at 60°C for 1 month, have proved less efficient than the Neusilin® US2 corresponding (Table 5).
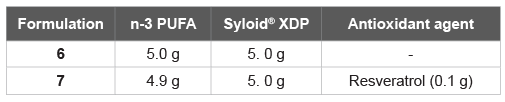
Table 6: Syloid® XDP-based formulation of fish oil
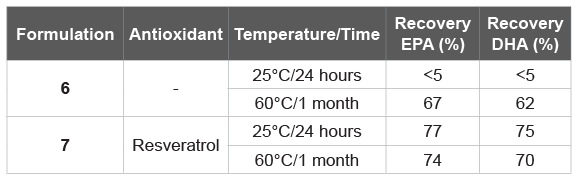
Table 7: Accelerated stability testing by radiation (25°C/24 h) vs high temperature (60°C/1 month) without and with resveratrol of Syloid® XDPbased formulation
The results presented here are the findings of a research project aimed at obtaining a powder of fish oil by adsorption on a solid matrix. It is well known, with fish oil as well as for all polyunsaturated fats, when absorbed on a porous solid support, due to a huge increase of the surface, their oxidation process is greatly accelerated facilitating also the self-oxidation (autoxidation). To this end, it was necessary to add antioxidant agents to ensure certain stability and prevent the degradation of polyunsaturated fatty acids. This technique, compared to the microencapsulation, leads to significant industrial advantages since the amount of oil adsorbed by the inert matrix is greater than 70%, the industrial production is not complex/expensive and the stability is quite high, even at temperatures of 30-40°C. It is important to highlight that not all antioxidant agents work efficiently as well as not all inert support materials are effective. Here we have showed that resveratrol, a natural antioxidant, was the best to stabilize fish oil avoiding the omega-3 fatty acids degradation in solid formulations; which becomes obvious by analysing the data of the Tables 1 and 6. Without antioxidant agents added, from data in Table 2, only after three months, at 25 and 30°C, EPA and DHA were significantly degraded, while the addition of resveratrol, Coenzyme Q10, α-tocopherol, and ascorbylpalmitate were able to improve the stability (Table 1). However, continuing to investigate on the stability over time and at high temperatures (Table 5), surprisingly only the resveratrol was able to maintain stable solid formulations containing omega-3 for longer periods. Furthermore, a direct proportionality between the antioxidant efficacy and the amount of resveratrol used was also observed: from 1% to 1.2%, after 3 months the recovery of EPA was significantly increased, and for both, EPA and DHA, maintained high even after six months.
A bioavailability study of the individual components, fish oil and resveratrol, toward the solid formulation with both adsorbed components in a solid matrix, it has not yet been fully investigated. Currently, a few studies on lab bench and in vivo are in progress. However, the result seems obvious. We already have some evidence that lead to this conclusion. When the powder (solid formulation) was suspended in water and the mixture left under mild stirring, for one hour at 37°C, it was observed the oil release from the solid matrix, which gradually moved towards the surface. The composition of this oil was the same as the starting oil (HPLC profile). Resveratrol, which was dissolved in the oil, was released in turn and it was found solubilised in water (HPLC analysis).
As for the in vivo bioavailability of the individual ingredients versus the solid formulation, we have previously carried out a study with statins solubilised in fish oil [16]. The bioavailability of the statins in oil was comparable to that of the statins administered as such, both administered orally on an empty stomach (unpublished data). Therefore, a similar behaviour is expected for fish oil and resveratrol.
When the oil was absorbed on an inert support, the typical smell of fish remains, in this study we also preliminarily tested a number of fragrances selected from Givaudan catalogues, usually used in food and pharmaceutical industry, to mask the smell. Results were good. At room temperature, the fish oil powders had no smell of fish. This was just a first approach but the result has been extraordinary. Taken together, these results suggest that such simple formulation is particularly suitable for industrial applications.
The authors claim to work as employees of sigma-tau IFR, S.p.A., an international pharmaceutical company wholly owned by and subject to the direction and coordination of Alfasigma S.p.A, which sponsored this research.
Download Provisional PDF Here
Article Type: Research Article
Citation: Santaniello M, Giannini G (2016) A Versatile and Stable Mixture of Fish Oil and Resveratrol in a Powder Formulation. Nutr Food Technol 2(3): doi http://dx.doi.org/10.16966/2470-6086.125
Copyright: © 2016 Santaniello M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access