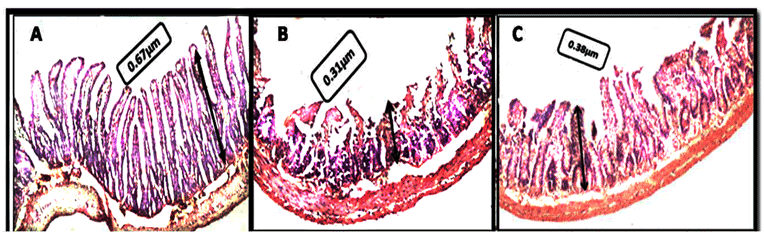
Figure 1: Photomicrographs showing villus height. A) Normal control group, B) irradiation control group exposed to 6 Gy irradiation, C) NSM pretreated group exposed to 6Gy irradiation, magnification 100X.


Nidhi Pandey1 Mohan Kumar2 S Pradhan3 A Mandal3 YB Tripathi1*
1Department of Medicinal Chemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India*Corresponding author: Prof Yamani B Tripathi, Department of Medicinal Chemistry, IMS, BHU, Varanasi-221005, India, E-mail: Yamini30@gmail.com
Radiation therapy is the most widely used treatment for cancer, but it causes the side effect due to intestinal damage. We examined radio-protective effect of methanolic extract of Nigella sativa (NSM) in albino rats after Total Body Irradiation (TBI). In the first experiment rats were treated with TBI at 4, 6, 10 Gy and mortality was recorded at 14th day and 6 Gy irradiated rats selected as experimental control group. After that assess the effect of NSM (200 mg/100 g bwt/day) on 6 Gy irradiated rats. NSM was orally given to rats 2 hrs before radiation and continued for 7th consecutive days. The changes in intestinal histology and antioxidant status were evaluated 7th day after sacrifice. NSM significantly enhance the antioxidant enzymes as compared to 6 Gy irradiated group. Evaluation of histological changes showed that NSM ameliorated intestinal morphological changes such as decreased villus shortening, mucosal erosion, and congestion and collagen deposition after 6 Gy irradiation in ileum. Moreover, the NSM treated group enhanced the survival time than the irradiated control group. Thus NSM had a protective effect on intestinal damage induced by radiation and decrease antioxidant enzymes. These results suggest that NSM is useful food supplement for preventing radiotherapy-induced intestinal damage in cancer patients.
Nigella sativa; Antioxidant; Radio-protective; Herbal; Food supplement
The attention has been given to the effect of radiation on the Gastrointestinal (GI) tract due to exposure of radiation after radiotherapy. Cancer patients undergoing radiotherapy have suffered from adverse effects related to the formation of free radicals, which cause oxidative damage to normal cells, including intestinal crypt cells [1]. When radiation dose is sufficiently high, leads to death of a majority of cells within the crypt, and subsequent loss of villi and severe damage to intestinal epithelium [2,3]. The Co60 radiation induces FR stress, which initiates inflammation and apoptosis in dividing cells. High doses of TBI or abdominal radiation similarly induce massive or complete loss of small intestinal crypts [4,5]. Radiation exposure to the abdomen attenuates bone marrow damage caused by radiation that has been suggested to sensitize mice toward lethality from Gastrointestinal (GI) syndrome [6]. GI syndrome is characterized clinically by anorexia, vomiting, diarrhea, dehydration, systemic infection, and, in extreme cases, septic shock and death [7]. Early histological changes include edema of the mucosa, hyperemia, capillary congestion, appearance of pyknotic nuclei and subsequent inflammatory reaction. These changes result to shortening of the villi, loss of epithelial cell lining, crypt damage, cell necrosis, edema in muscle-layer, high collagen deposition in the sub-mucosa and fibrosis. The time of survival following irradiation is strain-dependent [8]. This short survival time limits longterm studies of regenerative processes since it permits only assessment of early phases of crypt and epithelial regeneration. The typically short time of survival after high dose TBI or abdominal radiation is also problematic to study the impact of genetic manipulations that may delay, impair or prevent crypt regeneration/mucosal repair or to study long-term consequences of radiation such as fibrosis. The focus of irradiation protection has shifted to investigating the radio protective potential of natural products, including
plants and herbs, in the hope that suitable pharmacological agents, which protect humans against the deleterious effects of ionizing radiation in clinical and other conditions, can be identified. The herbal products have great potential to serve as radio protectors because they are rich cocktail of polyphenolic compounds, having significant free radical scavenging potential [9]. They may directly neutralize the FRs and some enhance the activity of endogenous antioxidant enzymes [10]. NS seeds in human and animals have shown it as immune modulator, anti rheumatism, anti diabetic, anti-mutagenic, analgesic, antiulcer, diuretic and antihypertensive, bronchodilator, antioxidant and hepatoprotective activities. Its seeds are rich in fixed oil, volatile oil, alkaloid, saponins, sterols and quinines [11]. Its polar extract contains Thymoquinone (TQ), one of its active ingredients [12]. Its polar methanolic extract also possesses antioxidant and anti-inflammatory property. Recently its radioprotection role has also been reported [13], but its role in protection of radiation induced intestinal damage is lacking. In the present work, we used a method of radiation that induces complete villi loss. We also compared NSM treated versus TBI model and demonstrated that NSM significantly improves survival, body weight recovery and normalization of intestinal villi after high dose abdominal irradiation.
Male rats of CF strain, 8-10 weeks old, weighing 75-100 g were purchased from the central animal house of Institute of Medical Sciences (IMS), Banaras Hindu University (BHU). They were provided a standard laboratory diet and water ad libtum. All experimental procedures were carried out in accordance animal ethics committee of our Institution (IMS, BHU-letter # Dean/2005-06/Animal Ethical Committee/390 dated-18.05.2006). The Unanaesthestized rats were positioned in well ventilated plastic boxes. The rats received TBI at 4, 6,10 Gy/ 4.6 min doses using 1.25 MV for the evaluation of intestinal injury.
The rats were subjected to TBI in different doses (4Gy, 6Gy, 10 Gy). Rats were maintained on normal chowed diet for 14days after radiation and noted that high mortality of 10 Gy radiated rats. 7 days survival was observed in 6 Gy radiated rats only. So experiment was performed in this model. At 4Gy no mortality observed at 7 days (Table 1). Tissue was harvested for histology at 7 days showed complete loss of villi following 6 Gy radiations in TBI. While 4 Gy showed insignificant change in villi (data not shown).
The NS seeds were purchased from local market and their authenticity was reconfirmed on pharmacognostical parameters by courtesy of Prof. K.N.Dwivedi, Department of Dravyaguna, Faculty of Ayurveda, IMS, BHU. The 100g dried seeds were crushed, powdered and exhaustively extracted with methanol in continuous soxhlet extractor. The solvent free extract was prepared by distillation and desiccation until constant weight was attained. The yield of methanolic extract (NSM) was 14% (w/w).The voucher specimen of this sample was preserved in the department wide reference noYBT/MC/14/1-2007.
Repeated doses of NSM was orally given to rats (50, 100, 200 mg/100 gm bwt) to each group, up to 14 day and survival time was determined.
Rats were divided into following three groups. Group I: control rats (without irradiation, fed 20% tween-20 in water), group II: radiation control (rats irradiated to 6 gy TBI), group III: rats pretreated NSM (50- 200 mg/100 gbw) followed by 6 Gy TBI. After that Animals were orally fed given concentration of NSM 200 mg/100 gbw) for 7 consecutive days followed by TBI. Animals were sacrificed at 7 days after last feeding of NSM to obtain intestine.
Intestine homogenate were used for assessment of antioxidant enzymes i.e Superoxide Dismutase (SOD) activity was measured using a Beauchamp and Fridovich (1971), catalase activity was measured by monitoring its ability to degrade H2 O2 ( Abei method) , Lipid Peroxidation Levels (LPO) was assessed by TBARS assay (Masugi and Nakamura;1976).
The entire small intestine was collected on ice, flushed with ice cold normal saline. The small intestine was divided into 3 segments corresponding to duodenum, jejunum and ileum. Tissues were opened longitudinally and fixed in fresh 10% formaldehyde in normal saline. Sections (<5 mm) were cut on a microtome and placed on microscope slides. Sections were stained with hematoxylin and eosin (H&E) to visualize villus morphology or with van- Geinson’s stain to assess collagen deposition. The slides were examined under microscope (Nikon, Tokyo, Japan) at 100X magnifications and quantitative changes were scored by using Nikon Imaging Software-Elements (NSBE). Quantitative data for villi height (in µm) and intact crypt numbers were quantified using 10X magnification. The quantitative data for the thickness of collagen layer (in µm) were obtained using 400X magnifications. as mean values ± standard deviation. All statistics analyses were unpaired t tests .P < 0.05 = *, P < 0.01 = **, P < 0.001 = ***, P >0.05. Asterisks indicate significant differences.
The radiation exposed rats showed the signs of radiation sickness within 2-3 days in dose dependent manner. Their food and water intake were reduced along with diarrhea and watering of eyes. 100% rats survived when they were placed on 4 Gy TBI, while none survived beyond day 7 when placed on 6Gy TBI and at 10 Gy none survived beyond 3 day (Table 1).
The repeated oral administration of NSM for 14-days showed 100% survival up to 200 mg/100g BW (Data not shown). Thus this dose was considered as optimum dose for all experiments.
Alteration in magnitude of lipid peroxidation and activity of antioxidant enzymes, the markers of oxidative stress, were studied in homogenate of intestine obtained from animals orally fed with NSM followed by TBI. The magnitude of lipid peroxidation was increased 98% in homogenate obtained from irradiated rats, which was significantly (P<.005) inhibited 41.3%. After 7 days of irradiation a significant decrease (p<0.001) in activities of SOD and catalase (43.5% and 28.7%) was observed in TBI animals. The protection of these antioxidant enzymes especially SOD and catalase in intestine of irradiated animals by NSM was higher 26.4% and 12.9% (P<0.05) than TBI rats (Table 2).
Intestinal segment was identifiable by H&E staining at day 7 postradiation.
S.N |
Dose of radiation |
Survival rate |
1 |
NORMAL | 14 ± 1.6 |
2 |
10Gy | 3 ± 0.8 |
3 |
6Gy | 7 ± 0.81 |
4 |
4Gy | 14 ± 0.7 |
Data presented as mean± SD. (n = 6)
Table 1: Optimization of dose of whole body Co60-irradiation to rats
S.N |
Group (N=6) |
Anti-oxidant activity |
||
SOD (Unit/mg |
catalase (Unit/ |
LPO (nmol/mg |
||
| 1 | Normal | 62.2 ± 2.0 |
52.2 ± 2.6 | 0.026 ± 0.007 |
| 2 | TBI (6Gy) | 34 ± 4.0 | 37.2 ± 2.4 | 0.46 ± 0.032 |
| 3 | 6Gy+NSM (200mg/g bwt) |
43 ± 5.7** | 42 ± 4.8** | 0.27 ± 0.04** |
Note: Rats were sacrificed on 7th day of TBI exposure. Data were expressed as mean ± SD. Values significantly different from control rats (6 Gy).
Table 2: Effect of NSM on 6 Gy TBI induced changes in activity of antioxidant enzymes in rat intestinal homogenate.
Compared to normal controls, significant decrease in the frequency of villi was noted in 6 Gy irradiated rats (12 ± 2.4 vs.21 ± 4.4), P<0.05. However, the NSM treatment (200 mg/100 g) significantly (P<0.05) prevented the reduction in villi frequency (Table 3 and Figure 1).
The height of villi was also decreased in experimental control (6 Gy TBI) rats as compared to normal rats (0.31±0.06 vs. 0.67±0.05), (P < 0.001) .However NSM treatment significantly (P < 0.05) prevented this reduction in the villi length (0.41±0.04 vs. 0.31±0.04), but it was lesser than normal control rats (Figure1).
In addition to frequency and height, the shape of villi was also distorted in TBI-rats. The NSM treatment significantly prevented this distortion in shape of villi (Figure 1).
Van Geinson’s staining revealed a consistent increase in serosal collagen deposition. 6 Gy TBI significantly increased (0.29 ± 0.04 vs. 0.12 ± 0.02), (P<0.001) the sub-mucosal deposition of collagen. However, the NSM treatment significantly (P<0.05) reduced (0.16 ± 0.012 vs. 0.29 ± 0.04) the sub-mucosal deposition of collagen as compared to experimental control (Table-3 and Figure 2).
Radiation Therapy (RT) or radiotherapy is a prime modality to treat solid tumours [14]. One of the major hurdles with respect to RT, is the preservation of normal tissue while still ensuring the effective killing of tumour cells [15]. The free radical mediated oxidative injury occurring after exposure of ionizing radiation (IR), damages both tumour as well as the normal cells, limiting the total radiation dose that can be administered [16]. For obtaining better tumour control therefore, it is of utmost importance to protect normal tissues against radiation-induced injury, particularly when higher doses of radiation are used [17]. It has been a longtime concern that antioxidant supplements, due to their ability to protect tissues from free radicals, could also protect cancerous tumors from the intended destructive effects of ionizing radiation when taken before or during treatment. The use of antioxidants during cancer treatment remains controversial. On the one hand, antioxidants might protect cancer cells against the oxidative damage induced by chemotherapy, which would mitigate against their use. On the other hand they may enhance druginduced cytotoxicity by blocking reactive oxidant species [18].
S.N |
Dose of |
Villi no. |
Length of villi |
deposition ( um) |
1 |
NORMAL | 21 ± 4.4 | 0.67 ± 0.07 | 0.12 ± 0.02 |
2 |
6Gy | 12 ± 2.4 | 0.31 ± 0.06 | 0.29 ± 0.04 |
3 |
6Gy+200NSM | 17 ± 1.6** | 0.41 ± 0.05** | 0.16 ± 0.012** |
Data were expressed as mean ± SD .Values significantly different from control rats (6 Gy).
Table 3: Effect of NSM treatment on TBI (6Gy) on histological changes in ileum

Figure 1: Photomicrographs showing villus height. A) Normal control group, B) irradiation control group exposed to 6 Gy irradiation, C) NSM pretreated group exposed to 6Gy irradiation, magnification 100X.
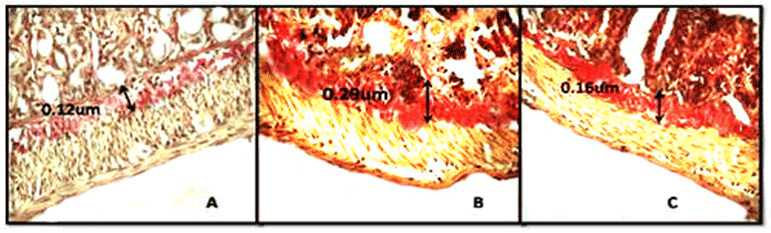
Figure 2: Photomicrographs showing effect of NSM on collagen deposition. A) Normal control group, B) irradiation control group exposed to 6Gy irradiation, C) NSM pretreated group exposed to 6Gy irradiation, magnification 400X.
People were given high doses of beta-carotene supplements to try to prevent lung and other cancers in smokers, the supplements were found to increase the risk of lung cancer [19]. Eating vegetables and fruits that contain beta-carotene may be helpful, but high-dose beta-carotene supplements should be avoided, especially by smokers [20].
Synthetic retinoids that are more potent than natural vitamin A or beta carotene have shown some ability to reverse pre-cancers in the cervix, mouth, throat, and skin [21]. They also may help prevent new tumors in people who have already been treated for these forms of cancer.
Antioxidants, when added adjunctively may improve the efficacy of chemotherapy and may prove to be safe [22].
Many studies have linked intake of foods rich in vitamin C to a lower risk of cancer [23]. But the few studies in which vitamin C has been given as a supplement have not shown a reduced risk for cancer [24]. More recently, vitamin C given through a vein (intravenously) has been found to have different effects than vitamin C taken in pill form. This has prompted renewed interest in the use of vitamin C as a cancer treatment [25].
Vitamin E supplements are not recommended to try to lower the risk of cancer or chronic diseases. Antioxidants N-Acetylcysteine (NAC) and vitamin E increase tumor cell proliferation by reducing ROS, DNA damage, and p53 expression in mouse and human lung tumor cells. Inactivation of p53 increases tumor growth to a similar degree as antioxidants and abolishes the antioxidant effect. Thus, antioxidants accelerate tumor growth by disrupting the ROS-p53 axis [26] in recent antitumor action of tocopherols were repoted [27].
Some new findings are antioxidants induce apoptosis in cancer cells and protect patients from painful side effects of radiation treatment may prove these compounds useful in future adjuvant therapy [28]. Studies suggest that people who eat more vegetables and fruits, which are rich sources of antioxidants, may have a lower risk for some types of cancer [29].
Several studies of antioxidant supplements have not found that they lower cancer risk. In fact, some studies have found an increased risk of cancer among those taking supplements [30]. To reduce cancer risk, the best advice at this time is to get your antioxidants through food sources rather than supplements [31].
Black seed oils are food. They are completely edible. Black Cumin contains beta-carotene, calcium, iron, sodium and potassium. It also has eight of the nine essential amino acids which our bodies cannot produce. In addition to the main active ingredients in black cumin, it also has thymoquinone, beta sitosterol. myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, arachidonic acid, protein, vitamin B1, vitamin B2, vitamin B3, folic acid, copper, zinc, and phosphorous . It is clear from the research that black seed oil and thymoquinone are both effective against cancer and do not have harmful side effects. It inhibits cancer cell activity and can even kill some types of cancer cells [32].
Black cumin seed oil and its extract thymoquinone have powerful benefits for various inflammatory diseases including liver cancer, melanoma skin cancer, pancreatic cancer, cervical cancer, breast cancer, bone cancer, stomach cancer, lymphoma, prostate cancer, colon cancer, and brain cancer [33].
It induces apoptosis, which means that it helps the body to systematically eliminate old cells, unneeded cells, and unhealthy cells (such as cancer cells) without releasing toxins into the body. It is earlier reported that mice were given black seed extract before being exposed to the gamma radiation. The results showed that the extract of black seed protected the liver, spleen, brain and intestines from gamma radiation damage for both the normal mice and the mice with tumors. It is concluded that the liquid extract of black seeds has protective effects against radiation-induced damage and biochemical alterations [34].
In one report rats with liver cancer were drink water that contained 0.01% thymoquinone. Rats that received thymoquinone did not develop liver cancer nodules, reduced liver injury markers and decreased tumor markers than the untreated group of rats. It is earlier reported thymoquinone’s ability to inhibit cancer cells from making clones of themselves and its ability to inhibit cancer cells from reusing cellular materials from other cells by means of autophagy, provide an exciting and emerging strategy for cancer therapy [35].
Our results indicate that polar methanolic extracts of NS seeds (NSM) significantly enhanced antioxidant properties of irradiated rats. This could be because of its high antioxidant potential as mentioned earlier. Our results also show its (Table 2), as it prevented the rise in radiation induced LPO in intestine tissue homogenate. The histological examination also showed prevention in irradiated induced loss of villi, shortening of villi height and collagen deposition (Figure1 and Figure 2).This is consistent with earlier reports related to TBI mediated changes in intestinal cells and villi [36-39]. The increase in collagen thickness (feature of fibrosis) is because of loss of the triple helix in collagen which is due to hydrogen abstraction through free radical mechanisms [40]. Thus NSM mediated reduction in collagen deposit may be attributed to its FR scavenging/ neutralizing potential. This could be presence of several phenolic compounds in NSM as reported earlier. Since the NSM has been given as pretreatment to TBI, there could be two possibilities. Either, the degree of damage is lesser, because the polyphenolic compounds of or it could be due to activation of the antioxidants enzymes in the Ilium cells. The presence of high polyphenolic content and high reducing power of NSM supports the 1st possibility of direct FR scavenging potential. The Thymoquinone (TQ) is one of the pure components of NS seeds, which is known free radical scavenger [41]. The TQ reacts with GSH, NADH and NADPH chemically and show antioxidant property [42]. However prevention in the TBI induced decline in activity of SOD and catalase in intestinal tissue homogenate indicates it role towards induction of endogenous antioxidant enzymes also. The rise in TBA-reactive Substances (TBARS) is due to oxidation of membrane lipids by FRs[43], altering the structure and function of cellular membranes.
However, with few exceptions, we can say that most studies have reported positive findings from the interaction of antioxidants during cancer treatment. Although further studies are needed, the predominance of evidence supports a provisional conclusion that dietary antioxidants do not conflict with the use of chemotherapy in the treatment of a wide variety of cancers and may significantly mitigate the adverse effects of that treatment.
Our results clearly indicate high TBARS in TBI rats and its prevention in NSM treated rats. It is concomitant with raised antioxidant enzyme activity in same tissue homogenate. Thus, it could be concluded that the methanolic extract of NS seeds possesses strong radio-protective property, which could be associate to its antioxidant activity. It could be its direct FR scavenging potential and also through activation of antioxidant enzymes.
This work was supported by Department of Atomic energy, Board of research in nuclear sciences (BRNS), Govt of India through as a research project is highly acknowledged.
The author(s) declare(s) that there is no conflict of interests regarding the publication of this article.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Pandey N, Kumar M, Pradhan S, Mandal A, Tripathi YB (2015) Effect of Methanolic Fraction of the Seeds of Nigella Sativa Linn on Radiation Induced GI Damage in Rats. Nutr Food Technol 1 (1): doi http://dx.doi.org/10.16966/2470-6086.102
Copyright: © 2015 Pandey N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access