Table 1: Comparison of AKI incidence rates in some developing
countries
a
Million child population
b
Million population
c
Studies conducted among children
d
Studies conducted among adult patients

Samuel N Uwaezuoke*
Honorary Consultant Pediatrician, Pediatric Nephrology Firm, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Nigeria*Corresponding author: Dr. Uwaezuoke SN, MBBS, FWACP, Honorary Consultant Pediatrician, Pediatric Nephrology Firm, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Nigeria, Tel: +2348033248108; E-mail: snuwaezuoke@yahoo.com
Acute kidney injury (AKI) in children is a major health problem worldwide particularly in tropical developing countries where the annual burden of AKI is estimated to be 11.3 million cases. The dismal outcome from AKI is evidenced by the high mortality rates recorded in many of these countries. The International Society of Nephrology (ISN) recently launched a global initiative which aimed to reduce preventable deaths from AKI to zero levels by the year 2025(0by25 initiative).
This review aims to highlight the epidemiology of pediatric AKI in developing countries and the mitigating factors to the 0by25 initiative.
Most of the etiologies of pediatric AKI are community-acquired single diseases such as malaria, gastroenteritis and hemolytic uremic syndrome. Thus, many cases of AKI in these regions are potentially preventable through community-based interventions such as malaria prevention and oral rehydration therapy. However, several factors may mitigate the realization of the goal of the 0by25 initiative in this part of the world. These medical, economic, socio-cultural and political factors should be addressed by the authorities if the ambitious goal of this initiative is to be achieved by the end of the next decade.
Pediatric AKI; Developing countries; Preventable deaths; 0by25 initiative
Acute kidney injury (AKI) remains a global health challenge with an estimated 13.3 million cases annually [1]. The picture is particularly worrisome in tropical developing countries where the yearly burden of AKI is estimated to be 11.3 million cases [1]. AKI is broadly classified into hospital-acquired and community-acquired AKI. Community-acquired AKI is more prominent in tropical developing countries whereas in the temperate developed world, AKI primarily develops in hospitalized patients [2,3]. The climate and environment in the tropics foster a plethora of community-acquired single diseases implicated in pediatric AKI such as malaria, gastro-enteritis, and haemolytic uremic syndrome (HUS) [2]. Notably, the epidemiology of AKI differs from country to country and from region to region [4]. Although the absence of renal registries in most developing countries has led to limited data on the overall epidemiology of AKI in these countries, its incidence has increased over the past few decades [5]. One report from India [6] has noted an increase in its overall incidence with evolving etiologic factors in the past 3 decades. The high mortality rates documented in many of these developing countries are a clear evidence of the dismal outcome from AKI [7,8].
The International Society of Nephrology (ISN) has recently launched a global initiative which aims to reduce preventable deaths from AKI to zero levels by the year 2025 with the acronym- 0by25 [1]. This initiative will be conducted in three key ways namely gathering evidence, creating awareness and education, and initiating an action plan.
This review aims to highlight the epidemiology of pediatric AKI in developing countries and the mitigating factors to the 0by25 initiative.
Using relevant search terms, information was gathered from Google, PubMed and Medline data bases including the ISN website, following a web search between January and June 2015. Articles that met the review objectives were selected; information was further obtained from crossreferences.
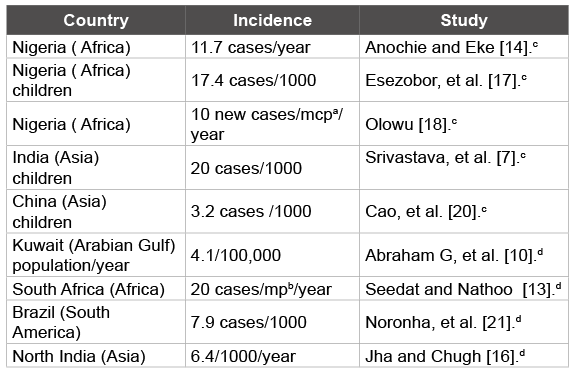
The global incidence of AKI is poorly understood because of underreporting, regional disparities, and differences in definition and case mix [9]. Data gathered from several developing countries reveal the challenges in defining the true incidence of AKI [4,10-19]. The obvious drawbacks in most of these studies include the absence of nationwide data collection systems and data from isolated centers not based on current definition of AKI [9]. In sub-Saharan Africa, a report from Southeastern Nigeria documented an incidence of 11.7 cases per year in a tertiary hospital [14]. The study was a retrospective review of database for all children from birth to 16 years admitted at the health facility over an 18- year period. The author’s case definition of ARF was ‘a rapid and progressive decline in renal function manifested as rising plasma urea and creatinine levels which are usually accompanied by oliguria (< 1 ml/kg/ hour) or occasionally polyuria’. Among the listed causes, gastro-enteritis and malaria were prominent. Elsewhere in South-western Nigeria, other workers reported a figure of 17.4 cases per 1000 children in another tertiary hospital [17]. In their study, a 2-year retrospective review of 4015 children aged 1 month and 16 years was conducted using the modified pediatric RIFLE criteria. Primary kidney diseases, sepsis and malaria were the common etiologic factors. In the same region, one author reported an incidence of 10 new cases per million children population per year in another tertiary hospital [18], where the major secondary etiologies of AKI were plasmodium falciparum malaria, septicemia, hypovolemia and obstructive uropathy [19]. In Asia, a study in India documented an incidence of 20 cases per 1000 pediatric admissions; the main causes of AKI were hemolytic uremic syndrome, sepsis, acute gastro-enteritis/ dysentery, glomerulonephritis and intravascular hemolysis [7]. In another Asian country, 388,736 Chinese pediatric patients admitted in 27 hospitals were studied with a reported incidence of 3.2 cases per 1000 children [20]. AKI was defined using the 2005 acute kidney injury network (AKIN) criteria. The three most common causes of AKI according to individual etiologic diseases were urolithiasis, acute glomerulonephritis and severe dehydration. Other reports from Kuwait in the Arabian gulf [10], South Africa [13], Brazil [21] and North India [16] documented incidences of 4.1 per 100,000 population per year, 20 cases per year per million population, 7.9 cases per 1000 hospital admissions and 6.4 per 1000 admissions per year respectively. These studies [10,13,16,21] were however conducted among adult patients (Table 1).
From the reports among the pediatric population in developing countries, most of the etiologies of AKI are preventable; this underscores the relevance of community-based interventions as potential tools in the reduction of AKI incidence rates and deaths.
The 0by25 initiative aims to eliminate preventable deaths from AKI worldwide by 2025 [1]. To achieve this goal, the mission of the initiative is to request for globally applicable strategies that allow timely diagnosis and treatment for AKI patients who present with potentially reversible diseases. This new global approach lays emphasis on developing countries in Africa, Asia and Latin America which harbor disadvantaged populations and have poor access to care. The goal will be achieved in three key ways. First, by gathering evidence, 0by25 will provide compelling new data to demonstrate the global burden of AKI, especially in low and middleincome countries with the aim of establishing AKI as a contributor to the Global Burden of Disease. The 0by25 initiative will compile existing and prospective data in order to better understand the prevalence of AKI and to improve diagnostic and treatment methods. Secondly, 0by25 will promote increased awareness of AKI across the global healthcare community, predominantly through education and training. Targeted information and education materials will be developed for a wide range of audiences, including healthcare professionals, patients and governments. Thirdly, 0by25 will work with those most affected to develop a sustainable infrastructure to enable “need driven” approaches to education, training and care delivery. Through pilot projects, 0by25 will implement globally applicable strategies that allow timely diagnosis and treatment of AKI for patients with potentially reversible diseases [1].

Table 1: Comparison of AKI incidence rates in some developing
countries
a
Million child population
b
Million population
c
Studies conducted among children
d
Studies conducted among adult patients
Interestingly, the first step of gathering evidence was successfully completed by the end of 2014 following the ‘Global Snapshot’- a prospective, cross-sectional study aimed at establishing the incidence of AKI in different settings around the world. Over 320 participating centers in more than 72 countries collected data from over 4,000 pediatric and adult patients with significantly new information from Africa, Asia and Latin America [1]. The results of this web-based data collection were unveiled at the ISN World Congress of Nephrology held in Cape Town, South Africa in March 2015. By the end of the next decade, it is hoped that the goal of zero preventable deaths from AKI will have been realized through the successive key steps. However, several factors may mitigate the realization of the goal of this initiative in developing countries; and these range from medical, political, socio-cultural to economic factors.
In these countries, AKI generally remains a disease of the pediatric age group [22-26]. The predominant etiologies are frequently associated with volume-responsive ‘pre-renal’ mechanisms [27,28], or toxic mechanisms [13,29]. Specific single diseases such as acute glomerulonephritis [30-33] and malaria [34,35] represent important causes of pediatric AKI. For instance, malaria continues to pose a health challenge with the upsurge in its global incidence [36]. Interestingly, the complications of severe falciparum malaria including AKI are simultaneously rising with the worldwide increase in the incidence of the parasitic infection [37]. In malaria endemic regions, the incidence of AKI may be 4% of malarial cases [38], but worldwide, its incidence varies between 0.6 and 60% of malarial cases depending on the geographical location [34]. Despite the global Roll-Back-Malaria program, preventive interventions have failed to meet the set target goals because of the enormous task of mosquito-vector control in tropical developing countries. The utilization of the long-lasting insecticidal nets and sustained environmental sanitation to curtail vector breeding sites has remained sub-optimal in these countries, especially in sub-Saharan Africa. Furthermore, the poor regulatory framework for the use of pharmaceutical products has encouraged widespread circulation of sub-standard antimalarial drugs resulting in drug- resistant plasmodium species. Secondly, diarrheal diseases remain among the top- killer diseases of under-five children in the developing world [39], partly as a result of delay in instituting appropriate rehydration therapy at home and in the health facilities. With the advent of oral rehydration therapy which promotes the use of oral rehydration salts and home-made fluids such as salt-sugar solutions, mortality and morbidity from the consequences of volume depletion (particularly from AKI) have substantially decreased [6]. Nevertheless, several studies in these countries still indicate low utilization rates for oral rehydration solution among primary caregivers [40-42]. As a result, reduction in AKI incidence rates, as well as its preventable mortality may not be sustained if the trend continues. Obviously, there should be renewed efforts by these countries to maintain the community-based interventions if preventable deaths from pediatric AKI are to be significantly minimized. Unfortunately, non-commitment by the authorities has continued to undermine the goals of similar global initiatives which aim to reduce childhood morbidity and mortality; this may also affect the 0by25 initiative.
Worse still, in these low-income developing countries, the cost of renal replacement therapy remains prohibitively high and unaffordable to majority of patients [18,23,43-46]. The poor access to renal replacement therapy is a major contributory factor to high AKI mortality rates in this part of the world, and may be a drawback to the goal of the 0by25 initiative. In addition, the upsurge in rebranding and unregulated marketing of herbal mixtures (a culturally-acceptable means of treatment in developing countries)may contribute to the rise in cases of AKI secondary to toxic mechanisms- well reported with these agents [13,29].
Most of the causes of pediatric community-acquired AKI in the developing world are preventable. Community-based interventions are therefore potential tools in the reduction of AKI incidence rates and deaths. Despite the 0by25 initiative, several mitigating factors may make its goal unrealizable in developing countries. These multi-faceted factors should be addressed by the authorities in order to ensure that the objective of this global initiative is met by the end of the next decade.
The author wishes to acknowledge the invaluable information obtained from the paper- Epidemiology of Acute Kidney Injury by Cerda J, et al. [9].
None to declare
Download Provisional PDF Here
Article Type: Review Article
Citation: Dr. Uwaezuoke SN (2015) Pediatric Acute Kidney Injury in the Developing World: How Realizable is the Goal of the Zero- PreventableDeaths- by- 2025 Initiative? Int J Nephrol Kidney Failure 2(1): doi http://dx.doi.org/10.16966/2380- 5498.121
Copyright: © 2015 Dr. Uwaezuoke SN. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access