Abstract
Purpose: Kidney transplant recipients (KTRs) are at high risk for de novo malignancies, and the incidence of prostate cancer (PCa) is about
2-fold higher in these patients than in the general population. Laparoscopic radical prostatectomy (LRP) is an accepted minimally invasive
treatment for organ-confined PCa. However, the procedure is challenging in KTRs because of the potential risk of allograft and ureteral injury. In
this study, we report our experience with LRP in patients following kidney transplantation.
Methods: Between 2006 and 2013, 234 consecutive LRPs were performed at Tokyo Women’s Medical University Hospital. We report the
outcomes for three patients with prior renal transplants who underwent retroperitoneal LRP.
Results: the mean age of the patients was 56.3 years. The average operative time was 236 min (range, 180–315 min). The mean estimated
blood loss was 54.6 mL, with no patients requiring blood transfusions. Although tension-free urethrovesical anastomosis was achieved in every
patient, anastomotic leakage occurred in two patients. The average hospital stay was 18.3 days, and the mean duration of urethral catheterization
was 21 days. Serum creatinine levels remained unchanged in two patients who had functioning renal allografts. The third patient commenced
hemodialysis postoperatively and resumed a continuous ambulatory peritoneal dialysis regimen two weeks after the operation.
Conclusion: Although technically challenging, retroperitoneal LPR remains an effective treatment option for localized PCa in patients who
have undergone kidney transplantation.
Keywords
Prostate cancer; Laparoscopic prostatectomy; Kidney transplantation
Introduction
Kidney transplant recipients (KTRs) are at high risk for de novo
malignancies. Genitourinary malignancies have been reported to represent
the second most common type of malignancy in the KTR population in
the United States. However, the incidence of prostate cancer (PCa) in renal
transplant recipients is not more than around two times that of the general
population. For clinically localized PCa, radical prostatectomy (RP) is the
standard treatment. Laparoscopic RP (LRP) is an accepted minimally
invasive treatment for organ-confined PCa. Robotic prostatectomy is also
accepted as a standard treatment for localized PCa. However, KTRs are at
risk for allograft and ureteral injury; therefore, sophisticated techniques
are required. We report our experience with LRP in KTRs and discuss
possible treatment choices for localized PCa in KTRs, especially surgical
management.
Patients and Methods
Between 2006 and 2013, 234 consecutive LRPs were performed at
Tokyo Women’s Medical University Hospital. Of the 234 patients, three
patients had previously undergone living donor kidney transplantation.
All three patients had American Society of Anesthesiologists Physical
Status 3. The patients’ preoperative serum creatinine levels were measured
on the day of surgery, and their postoperative levels were recorded on
the date of discharge. Pathological assessments were performed at our
institution, and the patients’ disease was staged using the 2002 tumor,
node, and metastasis (TNM) staging guidelines.
Surgical Procedure and Postoperative Management
Under general anesthesia, the patients were placed in the supine
position with their legs open. LRP was performed using five ports. The
retroperitoneal space was directly entered through a small subumbilical
incision and dilated using an endoscopic balloon dissection system
(PDB™
Balloon, Covidien Japan, Tokyo, Japan). The camera trocar was
placed, and abdominal pressure was maintained at 10 mmHg. The other
four ports were placed as shown in Figure 1. The patients were then placed
in a 15° Trendelenburg position. Adhesiolysis around the kidney allograft
was performed carefully to avoid damage to the transplanted ureter. RP
was then performed in accordance with the well-described technique of
endoscopic extraperitoneal RP [1,2]. After releasing the prostate from its
surrounding fatty tissue, the endopelvic fascia was sharply incised. The
puboprostatic ligaments were divided, and the dorsal venous plexus was
ligated using 2-0 Polysorb™
on an SH needle (Covidien Japan, Tokyo,
Japan) or an Endo-GIA™
Universal Stapler (Covidien Japan, Tokyo, Japan).
The bladder neck was incised using monopolar and bipolar electrocautery.
After the bladder neck was completely dissected and the anterior layer
of Denonvilliers’ fascia was incised, the vas deferens and seminal vesicles
were identified bilaterally. Both vasa deferentia were dissected, and the
seminal vesicles were mobilized. After incision of the posterior layer of
Denonvilliers’ fascia, the prostatic pedicles were identified and sharply
transected. Nerve-sparing surgery was performed from an intra- or
interfascial approach when indicated. Following complete mobilization of
the prostate, the urethra was divided using cold scissors. Once dissection
of the prostatic apex was completed, the prostate was retrieved with Endo
Catch™ (Covidien Japan, Tokyo, Japan) and temporarily placed next to
the camera trocar. Following posterior musculofascial reconstruction,
a watertight urethrovesical anastomosis was performed with a running
suture using 3-0 PDS II (Ethicon, Inc, West Summerville, NJ, USA) or
3-0 V-Loc™ with a 17-mm needle (Covidien Japan, Tokyo, Japan). The
first suture was placed at the 3 o’clock position. After completion of the
entire anastomosis, an 18 F Foley catheter was inserted. The watertight
anastomosis was confirmed by filling the bladder with 100 mL sterile
saline. Finally, a 15 F vacuum drain was placed in the pelvis. At the end
of the procedure, the specimen was removed through the camera port
wound. Immunosuppressive drugs were restarted on postoperative day
(POD) 1. The drain was removed between POD 2 and POD 5, when the
drain discharge became <50 mL per day. The Foley catheter was removed
and a voiding cystogram was performed on PODs 6–14.
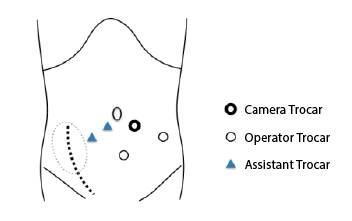
Figure 1: The 12 mm opticaltrocar is inserted using mini-laparotomy
technique. Four other trocars are placed under direct vision control so
as to avoid allograft kidney; a 10mm trocar in the left iliac fossa, another
10mm trocar in the midline between optical trocar and pubic bone and
two 5 mm trocars are in the right iliac fossa.
Case Reports
Case 1
A 52-year-old man with end-stage kidney disease caused by diabetic
nephropathy underwent ABO-incompatible living donor kidney
transplantation in 2007. The donor was his wife, and her kidney was
transplanted to his right iliac fossa. Laparoscopic splenectomy was
performed simultaneously as desensitization therapy. The patient’s
postoperative course was uneventful and the function of the allograft
was stable, with a serum creatinine level of 0.81 mg/dL. The patient’s
maintenance immunosuppressive protocol consisted of tacrolimus,
mycophenolatemofetil, and methylprednisolone. An annual health
check revealed a prostate-specific antigen (PSA) level of 16.0 ng/mL in
December 2007. Ultrasound-guided needle biopsy revealed left-sided
adenocarcinoma of the prostate (Gleason score 4 + 3). The estimated
prostate volume was 25 mL. The patient underwent retroperitoneal
LRP with left obturator lymph node dissection. The procedure was
completed successfully. The overall operative time was 316 minutes. The
prostatectomy and anastomosis required 190 and 100 minutes, respectively.
The estimated blood loss was 100 mL. There were no perioperative
complications. On POD 6, a voiding cystogram revealed leakage of the
contrast medium around the site of the anastomosis. The Foley catheter
was reinserted. On POD 8, the patient developed a high fever. Computed
tomography revealed a pelvic abscess around the anastomotic site and free
air in the transplanted ureter. Emergency laparotomy was performed on
the same day. The patient subsequently recovered without complications,
and he was discharged on POD 22. Histopathology revealed prostatic
adenocarcinoma in the left lobe with extracapsular extension at the apex
and a Gleason score of 4+5. The PSA nadir was 0.031, but PSA failure was
observed four months later. The patient received salvage external beam
radiation therapy (total dose, 64.8 Gy). Following radiation therapy, the
patient made steady progress. His PSA level is currently <0.01 ng/mL, and
his serum creatinine level is 0.7 mg/dL. Throughout the patient’s progress,
we reduced his immunosuppressants and did not change the drugs.
Case 2
A 52-year-old man presented to our hospital for a second kidney
transplant. He had received his first kidney transplant in 1997. The function
of the graft decreased nine years later, and the patient began peritoneal
dialysis therapy. Pretransplant cancer screening revealed a PSA level of
6.01 ng/mL. Prostate needle biopsy revealed left-sided adenocarcinoma of
the prostate (Gleason score 4+4). The estimated prostate volume was 41
mL. The patient underwent retroperitoneal unilateral nerve-sparing LRP
and left obturator lymph node dissection. The overall operative time was
180 min. The estimated blood loss was 30 mL. The patient commenced
hemodialysis on POD 2. The Foley catheter was removed on POD 4. The
patient was discharged on POD 6 and resumed his continuous ambulatory
peritoneal dialysis regimen two weeks after discharge. He underwent
successful living donor kidney transplantation three years later. At the time of
his most recent follow-up, the patient had no evidence of PSA relapse.
Case 3
A 63-year-old man presented with pollakiuria and urinary incontinence.
He had undergone successful kidney transplantation in November 1998
with a living related donor renal allograft to his right iliac fossa. The
allograft function was stable with a serum creatinine level of 1.54 mg/
dL. The maintenance immunosuppressive protocol was same as that of
the patient in Case 1. Fourteen years after the kidney transplantation,
digital rectal examination revealed a moderately enlarged prostate, and
the patient’s PSA level was 14.4 ng/mL. A prostate needle biopsy revealed
bilateral adenocarcinoma (Gleason score 4 + 4). The patient underwent
retroperitoneal LRP and left obturator lymph node dissection in August
2013. The operative procedure was the same as in Cases 1 and 2. The
total operative time was 215 min, and the estimated blood loss was 34
mL.The patient’s postoperative course was uneventful. However, on POD
7, a voiding cystogram revealed anastomotic leakage. The anastomosis site
leakage required 30 days to heal. On follow-up, PSA relapse was found to
have occurred 15 months after the prostatectomy. The patient received
salvage radiation therapy. Following radiation therapy, the patient has
displayed no evidence of recurrence. We reduced the immunosuppressants
postoperatively but did not change the drugs.
Discussion
The incidence of PCa is increasing year by year in Japan. PCa is the
top in male cancer at estimated morbidity in 2015. KTRs comprise a
population usually considered at high risk for malignancies, with an
estimated incidence that is 4–20-fold higher than that in the general
population [3]. However, it has been reported that the standardized
incidence ratio of PCa in KTRs is not very much higher than that in the
general population. The reported prevalence of PCa in renal transplant
patients ranges from 0.72 to 1% [4,5].
There are a variety of treatment options for localized PCa, including RP,
radiation therapy, and active surveillance. Local treatment of PCa in renal
transplant recipients is challenging, however, because they have renal
allografts in the iliac fossa, which were anastomosed to the iliac vessels and
the anterolateral wall of the urinary bladder. Active surveillance appears
inappropriate because KTRs are at higher risk of disease progression than
the general population. External beam radiation therapy can possibly cause
ureteral obstruction, which might enhance the risk of graft dysfunction.
The doses delivered to the ureteroneocystostomy have been calculated to
range from <20 Gy to >45 Gy depending on bladder repletion [6]. RP is
the gold standard in terms of therapeutic options for the management of
localized PCa in the non-KTR population, but it carries a risk of injury to
the renal graft, ureter, and bladder in renal transplant recipients.
Retropubic RP has been performed in selected renal transplant
patients, and good results have been achieved in many centers. Perineal
prostatectomy has also been reported to be successful [7]. The latter has
the advantage of avoiding direct manipulation of the renal allograft or
allograft ureter. LRP has advantages in that the magnified view enables
precise dissection, control of blood loss, and early patient recovery.
The first case of LRP was reported by Shah et al. in a 50-year-old renal
transplant patient with localized PCa [8]. They advocated a transperitoneal
approach because it avoids the adhesions present in the retroperitoneal
space surrounding the graft. There are several reports regarding LRP in
kidney allograft recipients. Most of the authors state that it is a technically
feasible and safe procedure without major complications and with no
different surgical challenges compared to the standard LRP. In contrast,
Robert et al. reported that there was a higher rate of rectal injury in KTRs
than in other patients, and iliac vein thrombosis resulted in graft loss [9].
Urethrovesical anastomosis can be more difficult because the renal allograft
can interfere with the movements of the instruments. Furthermore, lymph
node dissection on the ipsilateral side of the transplanted kidney is nearly
impossible. This is another limitation from the point of view of cancer
control. In our patients, we adopted an extraperitoneal approach. The
extraperitoneal approach has several advantages. It does not require either
a steep Trendelenburg position or high-pressure pneumoperitoneum,
which could affect renal allograft circulation during the operation.
This approach could also preserve peritoneal function and avert the
development of gastrointestinal complications. However, although the
extraperitoneal approach is ideal for patient safety, this procedure is more
technically challenging than the transperitonealapproach. Although
anastomosis leakage was frequently observed in our series, it was probably
caused by delays in wound healing associated with immunosuppressive
therapy, as opposed to being a technical problem.
Robot-assisted RP (RARP) appears to be the ideal surgical option for
localized PCa in renal transplant recipients because of its high flexibility
in instrument operation [10]. Jhaveri et al. reported the first case of
RARP [11]. Since 2012, the Japanese health insurance system has covered
RARP, and more than 200 RARPs have been performed, including a few
procedures that were performed in KTRs in our institutes. The specific
details of RARP are reported elsewhere.
In conclusion, although LRP is more technically challenging in KTRs
than in non-transplant patients, it remains a treatment option for localized
PCa in patients after kidney transplantation.
Article Information
Article Type: Case Report
Citation: Inui M, Iizuka J, Hashimoto Y, Takagi T,
Okumi M, et al. (2015) Management of Localized
Prostate Cancer by Retroperitoneal Laparoscopic
Radical Prostatectomy in Patients after Kidney
Transplantation. Int J Nephrol Kidney Failure 1(3): doi
http://dx.doi.org/10.16966/2380-5498.115
Copyright: © 2015 Inui M, et al. This is an
open-access article distributed under the terms
of the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 20 June 2015
Accepted date: 03
Sep 2015
Published date: 09 Sep 2015


