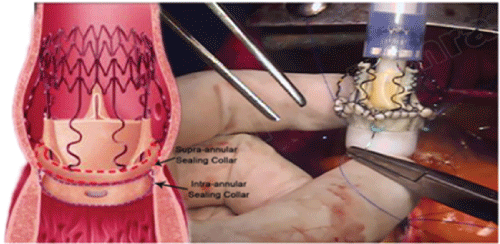
Figure 1: Intraoperative picture-A low profile valve with a large effective orifice area. Stability comes from a snug fit in the aortic root.


Maruti Yamanappa Haranal1* Shrinivas Hittalamani2 Sanjay Orathi Patangi2 Thasee Pillay1
1Department of Cardiothoracic and Vascular Surgery, Gleneagles Global Hospital, Bengaluru, Karnataka, India*Corresponding author: Haranal MY, Kausthubha Kuteera 9th cross, K R Layout, J P Nagar 6th phase, Bengaluru-560078, Karnataka, India, Tel: +91 9900972599; E-mail: marusurg@gmail.com
Recent advances in aortic bioprostheses involve deployment of suture less Aortic Valves. The sutureless Perceval aortic valve replacement (AVR) has been used in more than 20,000 cases worldwide and has excellent haemodynamics. Its other positive features include deployment in smaller aortic roots. In this case we describe an 82 year old lady presenting with significant symptoms attributable to a degenerating bioprosthesis. Previous surgery involved a bioprosthetic valve (21 Hancock bioprosthesis) and a Mitral Valve repair. Redo surgery and concomitant procedures are better served with a suture less AVR which can be deployed rapidly and presenting a larger effective orifice area. These characteristics also make minimal access surgery more feasible.
Sutureless AVR; Small Aortic Root; Redo surgery
There are currently two suturesless Aortic bioprostheses available. These include the Intuity valve from Edwards (California, USA) and the Perceval valve (LivaNova, London, UK). The latter has 10 year clinical experience with more than 22,000 implants worldwide. Clinical data is highly promising. This is borne out by the first clinical data at 5 years [1]. A consensus working group has further defined the indications and contra-indications for its use [2].
Particular advantages are the reproducibility of the haemodynamics, ease of implantation, reduced invasiveness of the procedure, shorter ICU and hospital stays. Lower blood transfusion and lower costs compared to Transcatheter aortic valve replacement (TAVI) is also present [3].
An 82 year old lady underwent Aortic Valve Replacement (AVR) with a 21 mm Hancock bioprosthesis 8 years previously. In addition, she had a repair of a regurgitant Mitral Valve. Other co-morbidity included ischaemic heart disease for which she had a coronary stent to the Right coronary artery (RCA). She also had a permanent pacemaker following her initial AVR. Presenting symptoms included breathlessness (NYHA II/III) and lethargy. This was progressive and restrictive. Clinically she was stable on anti failure medication. 2D Echo showed a dysfunctional aortic bioprosthesis that presented a gradient of 120 mmHg across it. Ventricular function was preserved. Pulmonary Artery (PA) pressure was moderately elevated. The Chest Radiograph revealed cardiomegaly. Other investigations were satisfactory
A chest Computerized Tomogram (CT) scan was carried out to exclude significant calcification in the ascending aorta. This showed calcification in the arch and descending aorta. The Sinotubular (ST) to annulus ratio was less than 1.3.
A decision to proceed with AVR was made with the patient and her family. Options of TAVI were considered as were conventional and sutureless AVR. The decision to proceed with a sutureless AVR was made after presenting the risks and benefits of the various options.
An intra-operative Trans Esophageal Echo (TEE) confirmed a diseased bioprosthesis with only mild Mitral regurgitation. The gradient across the valve was 120 mmHg. The ST junction to annulus ratio was measured as 1.14:1.
Surgery was commenced with exposure of the right femoral artery and the vein. The sternum was opened using an oscillating saw. The heart was partially freed. The aorta, the right atrium and the right superior pulmonary vein were completely freed. Cannulation of the ascending aorta and the right atrium allowed the commencement of Cardiopulmonary Bypass (CPB). A left ventricular vent was passed via the right superior pulmonary vein (RSPV). A cardioplegia cannula was placed into the ascending aorta.
After cross clamping and delivery of cardioplegia into the aortic root, a high transverse aortotomy was made. The degenerated Hancock prosthesis was explanted. Further debridement of the annulus was carried out. The mitral valve was observed to be close to the aortic annulus and care had to be taken to preserve its integrity.
The native aortic annulus admitted a large Perceval sizer (equivalent to a 23-25 valve). Using 3, 4/0 Polypropylene sutures placed at the nadir of each sinus, further sizing was carried out. The 3 sutures were deemed to be 120 degrees apart. A valve was collapsed on the side table. The 3 sutures were passed through 3 eyelet sutures on the collapsed valve (Figure 1). The valve was deployed, a balloon was used to re-expand it and an inspection of the valve position was carried out (Figure 2). The aortotomy was closed. After thorough de-airing the cross clamp was released. Spontaneous cardiac activity commenced, in heart block. The PPM was recommenced. After filling an effective ejection, the valve was inspected by TEE. This showed excellent valve function with no para-valvular leak and a mean gradient of 2 mmHg (Figure 3).

Figure 1: Intraoperative picture-A low profile valve with a large effective orifice area. Stability comes from a snug fit in the aortic root.
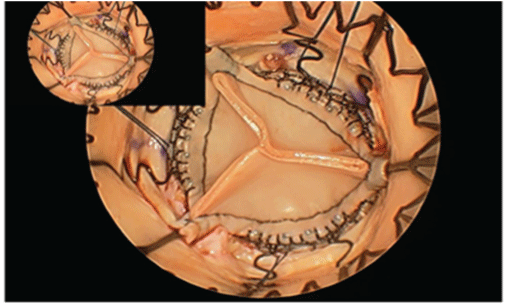
Figure 2: Intraoperative picture-The properly deployed valve mimics a native valve closely. The lack of a sewing cuff aids haemodynamics.
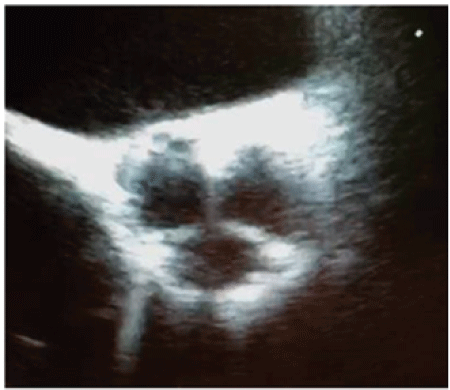
Figure 3: TEE-Mid-esophageal short axis view-A short axis TEE reveals a prosthesis that is very similar to a native valve.
The post-operative period was uneventful; the patient was transferred to the ward on the 2nd day and discharged home on the 5th day. Post-operative echo showed equally good haemodynamics to the intra-operative TOE.
Transcatheter Aortic valve Implantation (TAVI) has made AVR possible in the higher risk group of patients. Costs associated with this therapy are however a deterrent. Sutureless valves have also been introduced [4]. The three valves introduced onto the market include the Enable (3F), Perceval (LivaNova) and Intuity (Edwards). The Enable has since been withdrawn.
Transcatheter Aortic Valve Implantation (TAVI) has added to the surgical therapies available for severe aortic stenosis. However, concerns about durability, haemodynamics, subclinical thrombosis and occult intra-cerebral bleeding should be taken into context. The price also presents limitations to applicability
The Perceval valve offers as less invasive a procedure as possible, with the advantages of great haemodynamics and satisfactory durability. Its unique design of proven pericardial leaflets mounted on a Nitinol stent allow for a wide range of indications in patients with severe aortic stenosis or mixed aortic valve disease.
The Perceval valve remains a widely used valve with more than 22, 000 implants worldwide. It has attained FDA approval in January 2016. The Perceval valve is easy to implant reducing the complexity even in challenging and time consuming procedures. It is safe and is therefore a valuable option to reduce post-operative complications. Perceval valve allows reproducible results both in Minimal Invasive Cardiac Surgery [5] and conventional sternotomy.
The indications for use are aortic stenosis or stenoinsufficiency. Contra-indications include aneurysmal dilatation of the aortic root and bicuspid aortic valve. Due to the design of the valve, the ratio of the Sinotubular (ST) junction to the aortic annulus must not exceed 1.3.
The main categories of patients that will benefit from sutureless AVR are:
However, due to the lack of a sewing ring and cuff, the increased effective orifice area offered by a Perceval valve makes the valve a suitable alternative in any patient requiring a bioprosthesis in the aortic position. Gradients in single figures characterize this valve. The gradients tend to be lowest around 6 weeks following implantation.
Questions about the durability remain to be answered but initial clinical studies indicate extremely favourable valve function out to 7-10 years. The need for a permanent pacemaker (4.2% VS 2.2%) is higher than conventional AVR [7] but not as high as TAVI. Some report a transient thrombocytopenia [8] but this is not consistent in all studies.
Our patient certainly benefited from the Perceval valve. It resulted in a short cross clamp and CPB run, it allowed a larger valve and conferred excellent haemodynamics. Whilst this case illustrates the use of the valve in a small lady, its use in extremely large patients is also beneficial. In the latter group, presenting as large an effective orifice area as possible accelerates left ventricular mass regression in these patients with left ventricular hypertrophy. In addition, the possibility of patient prosthesis mismatch is reduced. In concomitant procedures with anticipated long cross clamp times, the use of the Perceval valve is particularly indicated.
The Perceval bioprosthesis is a relatively new innovation in Cardiac Surgery. Long term results are awaited. Initial results are highly encouraging and its applicability in day to day aortic valve replacement has already been demonstrated.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from the individual participant included in the study
The authors declare that no conflict of interest exists.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Haranal MY, Hittalamani S, Patangi SO, Pillay T (2018) Sutureless Aortic Valve Replacement in a High Risk Octagenerian. J Hear Health 4(1): dx.doi.org/10.16966/2379-769X.144
Copyright: © 2018 Haranal MY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access