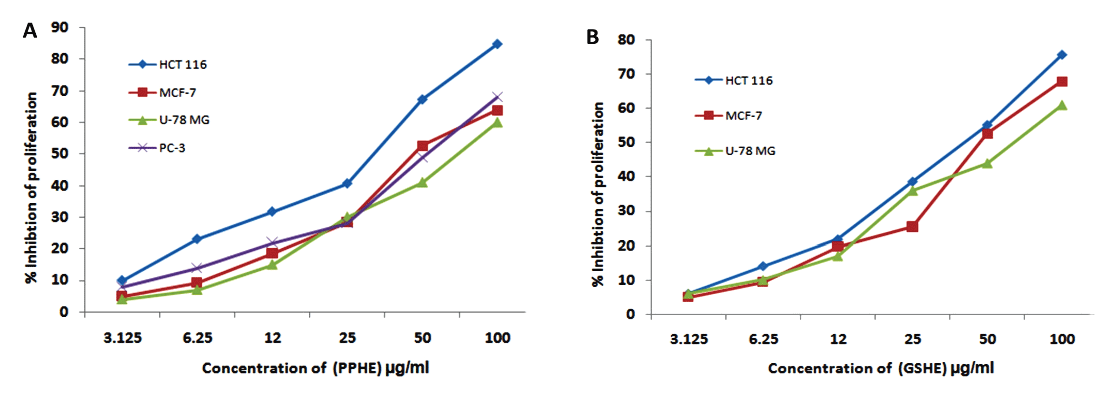
Figure 1: Dose dependent inhibitory effect of the active extracts of the selected fruits extracts against carcinoma cell lines (A) PPHE (B) GSHE.


Saad Sabbar Dahham1* Yasser M Tabana1 Loiy EA Hassan1 Mohammed O Ezzat2 Nik Noriman Zulkepli3 Amin M Shah Abdul Majid1
1EMAN Research, and Testing Laboratory, School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang, Malaysia*Corresponding author: Saad Sabbar Dahham, EMAN Research, and Testing Laboratory, School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang, Malaysia, Tel: 0060176988685; E-mail: hawk_dijla@yahoo.com
Several epidemiological studies have suggested that the Mediterranean dietary pattern, which includes consumption of fruits, has been linked to lower risk of various forms of cancer. This study assessed the antiangiogenic, antioxidant, and antiproliferative properties of extracts from common Mediterranean fruits including, dates (Phoenix dactylifera), figs (Ficus carica), grapes (Vitis vinifera), olives (Olea europaea), and pomegranates (Punica granatum).
Different parts of the fruits were extracted with two organic solvents as well as water. Twenty-one extracts were screened for antiangiogenic activity using the rat aorta ring assay. They were tested for cytotoxic activity using four cancer cell lines and one normal cell line, and their antioxidant properties were measured using two methods (2,2’-diphenyl-1-picrylhydrazyl radical (DPPH) and ferric reducing antioxidant power (FRAP) assays). GC-MS analysis indicated the presence of different phytochemical classes in fruits, phenolic acids, sterols, flavonoids, fatty acids, triglycerides, and alkaloids are the main constituents in these fruits. Of the 21 extracts tested, 14 exhibited substantial antiangiogenic effect. The ethanol extracts of pomegranate and olive had the highest activity (100% inhibition at 50 µg/mL), as did the hexane extract of the grape. Likewise, the hexane extract of pomegranate peel and grape seed had the highest cytotoxic effects and was found to induce apoptosis in the colorectal cancer cell line HCT-116. Furthermore, the extracts prepared from ethanol had the highest antioxidant activity among the tested fruits, pomegranate peel displayed potent antioxidant activity, with the lowest IC50 value calculated (6.06 µg/mL). The study demonstrated considerable anticancer and antioxidant properties in Mediterranean fruit extracts.
Antioxidant; Mediterranean fruits; Antiangiogenic; Phytochemical; Cytotoxic
Cancer remains a leading cause of mortality and morbidity worldwide. However, despite the extensive advances that have been made in cancer therapy, chemotherapy side effects is still the major dose limiting toxicity. Poor therapeutic effect and unaffordability have led to the increase demand for alternative anticancer agents derived from natural products [1]. Today, there is a remarkable progress in the utilization of medicinal plants and their derivatives for the prevention and treatment of human cancer. Approximately, there are more than 300,000 flowering plants that have been recorded and identified worldwide, out of which, about 60% of the plants are being used for drug development [2]. It is common practice in alternative and complementary medicine studies nowadays to find active plant products in a composite formula for certain disease and further explore active ingredients, thereafter composite formula can be standardized for biological activity and chemical composition [3]. Fruits are one of the ancient sources of food, and they have not altered much throughout the history of man. There are several historical evidences associating fruits with eternal life in ancient Sumerian, Babylonians, and Egyptian cultures, and they also are significant in Jewish, Christian, and Muslim traditions [4,5]. As estimated 35% of cancer deaths may be attributable to dietary and nutritional factors [6]. On the other hand, the regular consumption of fruits and vegetables appears to prevent about 25% of colon cancers, 15% of breast cancers, and 10% of prostate, pancreatic, and endometrial cancers [7]. Modern clinical and experimental studies show that high intake of fruits contributes to reduced risk of certain types of human cancers [8-10].
The major phytochemicals derived from fruits that have potential to prevent cancer includes carotenoids, vitamins, terpenoids, silymarin, quercetin, indole-3-carbinol, and sulphoraphane [6]. These phytoconstituents usually target multiple cell signaling pathways of cancer, they are safe, and they lack the side effects of conventional chemotherapies.
The natural pigments that give fruits their color are an excellent source of natural antioxidant molecules, such as flavonoids. In addition, the phenol, anthocyanin, and ascorbic acid content of small fruits contribute extensively to the antioxidant complement of some fruits [11]. Among the colorful fruits, (date, fig, grape, olive, and pomegranate), are consumed as a part of the Mediterranean diet, which has received considerable attention in recent years due to its health benefits [12].
It is now commonly believed that the complex mixture of phytochemical components existing in a diet consisting of 5–10 servings of fruits per day is more effective than their individual pure compound in cancer prevention due to both additive and synergistic properties [12].
Chemoprevention by ingestion of edible phytochemicals suggests an applicable, acceptable, and accessible avenue for cancer prevention and management. With the side effects of cancer therapy and the burden of healthcare costs, alternative programs are urgently needed to establish new cancer prevention approaches for general public health [13]. Hence, it is vital to examine potential anticancer activities of whole fruit extracts containing all phytoconstituents extracted by different solvent systems.
In the present study, we generated extracts of five popularly consumed fruits (dates, figs, grapes, olives, and pomegranates) using two organic solvents as well as water. The goals of this study were to: (i) evaluate the antioxidant activity of selected fruit extracts; (ii) evaluate their ability to inhibit the proliferation of human breast (MCF-7), brain (U-78 MG), colon (HCT-116), and prostate (PC-3) tumor cell lines as well as a normal cell line (human umbilical vein endothelial cell (HUVEC) line and elucidate their mechanism of action; (iii) determine their antiangiogenic effect using the rat aortic ring assay; and (iv) investigate the phytochemical profile of the active extracts using gas chromatography-mass spectrometry (GC-MS).
All cell cultures and their reagents were purchased from Gibco (USA). Phosphate buffered saline trypsin, DMSO, ethanol, methanol, n-hexane, DPPH and MTT 3-(4 5-dimethylthiazol-2-yl)-2 5-diphenyltetrazolium bromide) were purchased from Sigma (Germany).
Five fruits (dates, figs, grapes, olives, and pomegranates) were assessed in this study. Fruits were collected during the period April 2015 from Istanbul, Turkey. Taxonomical identification was confirmed by Mr. V. Shanmogan, a senior taxonomist. A herbarium samples were submitted at the Department of Botany, School of Biological Sciences, Universiti Sains Malaysia, Pulau Pinang, Malaysia.
The fruit materials were dried in an oven (35–40°C) and powdered mechanically. The pulverized plant material (50 g) was subjected to sequential extraction starting with n-hexane and followed by ethanol and water. All extracts were prepared with 250 mL of the solvent using the hot maceration (40°C) method with intermittent shaking. The extracts were filtered and concentrated at 45°C under vacuum using a rotary evaporator (Buchi, USA) and further dried overnight at 45°C. Stock solutions of the extracts were prepared at 10 mg/mL in 100% DMSO. Further serial dilution of the stock was performed with cell culture media to obtain a range of desired concentrations of the extracts.
Quantitative chemical analysis of the active fruit extracts was conducted using GC-MS to identify the active compounds that might be responsible for the antioxidant, antiproliferative, and antiangiogenic activities of the extracts. The assay conditions were as follows: HP-5MS capillary column (30 m × 0.25 mm, ID × 0.25 µm, film thickness); held at 70°C for 2 min, raised to 285°C at a rate of 20°C/min and held for 20 min; 285°C for the MSD transfer line heater; carrier helium at a flow rate of 1.2 mL/min; 2:1 split ratio; 1 µL solution of fruit samples in hexane or/and ethanol (10 mg/mL) was injected automatically. The scan parameter for low mass was 35, and for higher mass it was 550. The constituents were identified by comparison with standards using NIST 02. A total ion chromatogram was used to compute the percentage of the identified constitutes.
DPPH scavenging effect of extracts was investigated as described by Tabana YM, et al. [14]. Briefly, stock solution of DPPH was prepared in methanol at 200 µM. A serial dilution of extracts was prepared in methanol to obtain (200, 100, 50, 25, 12.5 and 6.25 µg/ml). 50 µM DPPH reagents was added at final concentration and incubated at 30°C in the dark for 30 min. Consequently, absorbance was measured at 517 nm and DPPH scavenging activity was calculated using the formula (1-(absorbance of samples-blank)/(absorbance of negative control–blank)) × 100%. Median inhibitory concentration (IC50s) were calculated from the dose response curves (n=3). Methanol was used as a negative control.
The FRAP assay was assessed as described earlier [15]. The results were expressed as µM·Fe2+·mg−1. All measurements were carried out in triplicate and the mean ± S.D values were calculated.
The cytotoxicity assay was conducted according to the method previously described [16]. Cells were seeded at 1.5 × 104 cells in each well of a 96-well plate in 100 µL of fresh culture medium and were allowed to attach overnight. For screening, the cells (70–80% confluency) were treated with fruit extracts at 3.125, 12.25, 25, 25, 50 and 100 µg/ml concentrations to get the IC50. After 48 h of treatment, the medium was aspirated and the cells were exposed to MTT solution prepared at 5 mg/ml in sterile PBS. The solution was added to each well at 10% v/v and incubated at 37°C in 5% CO2 for 3 h. Absorbance was measured using the TECAN microplate reader at the primary wave length of 570 nm and the reference wavelength of 620 nm. The results were presented as a mean percent inhibition to the negative control ± S.D, (n=4).
The effect of fruit active extracts on nuclear chromatin condensation in HCT-116 cells was quantified by fluorescence microscopy using Hoechst 33258 stain [17]. HCT-116 cells were treated with the fruit extracts (50 µg) and analyzed separately at two different time intervals (6 and 12 h). DMSO (0.1%) was used as negative and. The cells were fixed in 4% paraformaldehyde for 20 min before staining with Hoechst stain (1 µg/mL in PBS) for 20 min. Nuclear morphology was examined under a fluorescent microscope. Cells with bright colored, condensed, or fragmented nuclei were considered to be apoptotic. The number of cells with apoptotic morphology was counted in randomly selected fields per well. The cells were photographed at 20 × magnification using an EVOS f1 digital microscope (Advanced Microscopy Group, USA). The apoptotic index was calculated as percentage of apoptotic nuclei compared to the total number of cells and was presented as the mean ± SD (n=6).
This assay was carried out following [18], with slight modification. Thoracic aortas were removed from euthanized male Sprague-Dawley rats (12–14 weeks old), rinsed with serum-free medium, and cleared of fibro-adipose tissues. The aortas were cross-sectioned into small rings (approximately 1 mm thickness) and seeded individually in 48-wells plate in 300 µL of serum-Absorbance was measured by infinite® Pro200 TECAN Group Ltd., (Switzerland) free M199 medium containing 3 mg/ mL fibrinogen and 5 mg/mL aprotinin. Ten microliters of thrombin (50 NIH U/mL in 1% bovine serum albumin in 0.15 M NaCl) were added to each well and incubated at 37°C for 90 min to solidify. A second layer (M199 medium supplemented with 20% HIFBS, 0.1% aminocaproic acid, 1% L-glutamine, 2.5 µg/mL amphotericin B, and 60 µg/mL gentamicin) was added into each well (300 µL/well). Fruit extracts were added at a final concentration of 100 µg/mL. Suramin and DMSO (1%) were used as positive and negative controls, respectively. On day 5, the aortic rings were photographed at 4 × magnification by using an inverted light microscope supplied with a digital camera. The magnitude of microvessels outgrowth from aortic tissues was then quantified according to a previously reported technique [18]. The distance of growth from the primary tissue explants was measured by Leica Quin computerized imaging system. The distance of growth of microvessels in twenty growth points selected at regular intervals around the ring was measured and the average was calculated. The results are presented as mean percent inhibition ± SEM (n=6).
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Statistical significance was considered at p<0.05 and p<0.01 and were indicated as * and **, respectively.
Table 1 shows the list of fruits and fruit parts evaluated in this study. Three exacts were prepared from each part of each fruit, starting with n-hexane followed by ethanol and then water. The yield of each extract was calculated and is presented as w/w percent yield (Table 1). The hexane extracts of all tested plant materials produced the lowest yield, except for the red grape skin extract (2.6%), whereas the water extracts had the highest yield, and those of the ethanol extracts fell in between. The highest yield was 4.30% for the water extract of pomegranate peel.
Table 1 also shows the radical scavenging ability of fruit extracts as determined by the DPPH and FRAP assays. In general, the extracts prepared from ethanol had the most potent antioxidant activity, whereas the extracts prepared from the solvent hexane displayed poor or moderate DPPH and FRAP scavenging activity. Among the tested fruits, pomegranate peel exhibited potent (p<0.01) antioxidant activity, with the lowest IC50 value calculated for the ethanol extract (6.06 µg/mL). Similarly, the ethanol extract of the grape skin and olive skin showed significant antioxidant activity (p<0.01), with IC50 values of 9.60 and 10.11 µg/mL, respectively. The ethanol extract of pomegranate peel and olive skin demonstrated remarkable FRAP radical scavenging effect, with IC50 values of 10.20 and 13.21 µM of Fe2+/mg, respectively (Table 1). The n-hexane extracts of the tested fruit parts displayed either moderate or insignificant DPPH scavenging activity, except for the hexane extract of grape skin, which demonstrated potent free radical scavenging activity (IC50=18.35 µg/mL).
Fruits |
Part used |
Solvent |
Yield (%) |
DPPH |
FRAP |
Dates |
Fruit |
n-hexane |
1.50 |
120.05 ± 1.12 |
33.13 ± 1.23 |
Ethanol |
1.93 |
18.34 ± 0.52* |
43.06 ± 1.53 |
||
Water |
2.55 |
33.12 ± 0.69 |
38.09 ± 0.59 |
||
Fig |
Fruit |
n-hexane |
1.10 |
70.50 ± 0.83 |
120.90 ± 0.88 |
Ethanol |
2.8 |
16.09 ± 0.80* |
20.42 ± 1.22* |
||
Water |
3.7 |
22.10 ± 0.35 |
50.30 ± 1.42 |
||
Grape |
Skin |
n-hexane |
2.06 |
18.35 ± 0.59* |
163.01 ± 1.31 |
Ethanol |
1.95 |
9.60 ± 0.25** |
95.20 ± 1.17 |
||
Water |
3.55 |
16.25 ± 0.93* |
71.33 ± 1.25 |
||
Seed |
n-hexane |
1.22 |
32.46 ± 0.55 |
40.61 ± 0.43 |
|
Ethanol |
1.95 |
11.53 ± 0.12* |
22.03 ± 0.55* |
||
Water |
0.9 |
22.50 ± 0.15 |
66.89 ± 0.49 |
||
Olive |
Skin |
n-hexane |
0.45 |
90.10 ± 0.65 |
21.41 ± 0.51* |
Ethanol |
1.00 |
10.11 ± 0.22** |
13.21 ± 0.23** |
||
Water |
2.34 |
13.09 ± 0.71* |
33.02 ± 0.57 |
||
Pomegranate |
Peel |
n-hexane |
1.32 |
53.49 ± 0.21 |
26.76 ± 1.10 |
Ethanol |
1.14 |
6.06 ± 0.83** |
10.20 ± 0.25** |
||
Water |
4.30 |
12.80 ± 0.20* |
27.54 ± 0.88 |
||
Seed |
n-hexane |
0.69 |
67.92 ± 0.26 |
160.09 ± 1.31 |
|
Ethanol |
1.50 |
23.30 ± 0.33 |
88.52 ± 0.19 |
||
Water |
1.99 |
34.06 ± 0.19 |
73.11 ± 1.43 |
Table 1: Yield and antioxidant activity of different extracts of the selected fruits.
The values are expressed as mean ± SD (n =3). Statistical significance is expressed as *=P<0.05, **=P<0.01.
Fruit extracts were tested for their ability to inhibit the growth of HCT-116, U-78 MG, MCF-7, and PC-3 tumor cell lines as well as normal HUVEC cells (Table 2). The extracts showing >60% inhibition of cell proliferation were considered to be active extracts. Hexane extracts of pomegranate peel exhibited the highest cytotoxicity to all tested cancer cell lines, whereas the hexane and ethanol extracts of fig fruit and grape seed showed selective antiproliferative effects against HCT-116, with inhibition of 61.43% and 74.01%, respectively. U-78 MG cells were susceptible to the hexane extract of date fruit and pomegranate peel, with inhibition of 61.12% and 60.22%, respectively. The ethanol extract of grape seed showed selective cytotoxicity to HCT-116, PC-3, and MCF-7 with inhibition of 65.33%, 62.52%, and 60.03% respectively. Further, the most active extracts were selected to illustrate the dose-response cytotoxic activity. The median inhibitory concentration (IC50) values for the most active extract of grape seed hexane extract (GSHE) and pomegranate peel hexane extract (PPHE) were calculated for all tested cell lines and the values are given in table 3 .The dose-response curves were depicted in figures 1A and 1B. Overall, the tested cancer cell lines showed various degrees of susceptibility to different fruit extracts, whereas all of the fruit extracts showed poor cytotoxicity against the normal cell line.
Fruits |
Used part |
Solvents |
% inhibition of cell proliferation |
||||
HCT-116 |
MCF-7 |
U-78 MG |
PC-3 |
HUVEC |
|||
Date |
Fruit |
n-hexane |
58.60 |
39.20 |
61.12 |
17.43 |
6.43 |
Ethanol |
46.23 |
22.31 |
49.94 |
22.31 |
4.50 |
||
Water |
17.93 |
7.66 |
44.50 |
15.09 |
3.05 |
||
Fig |
Fruit |
n-hexane |
61.43 |
50.10 |
48.32 |
55. 11 |
7.70 |
Ethanol |
47.85 |
34.06 |
32.77 |
36.81 |
5.84 |
||
Water |
38.09 |
30.15 |
27.45 |
31.20 |
5.55 |
||
Grape |
Skin |
n-hexane |
55.20 |
63.89 |
51.33 |
55.41 |
8.50 |
Ethanol |
58.10 |
43.74 |
50.91 |
59.55 |
10.51 |
||
Water |
48.12 |
52.15 |
43.17 |
37.21 |
7.33 |
||
Seed |
n-hexane |
74.01 |
65.16 |
60.50 |
55.10 |
15.60 |
|
Ethanol |
65.33 |
55.03 |
39.80 |
62.52 |
9.03 |
||
Water |
39.40 |
22.50 |
25.52 |
40.12 |
6.20 |
||
Olive |
Skin |
n-hexane |
63.40 |
55.10 |
46.60 |
51.00 |
7.41 |
Ethanol |
39.05 |
30.71 |
41.12 |
38.30 |
5.99 |
||
Water |
22.80 |
10.00 |
23.05 |
9.71 |
3.08 |
||
Pomegranate |
Peel |
n-hexane |
83.98 |
62.55 |
60.22 |
68.52 |
14.50 |
Ethanol |
65.10 |
57.09 |
55.95 |
60.10 |
10.20 |
||
Water |
41.80 |
38.15 |
38.40 |
44.37 |
5.91 |
||
Seed |
n-hexane |
52.64 |
60.23 |
58.90 |
50.31 |
16.03 |
|
Ethanol |
42.10 |
51.19 |
55.00 |
42.29 |
8.52 |
||
Water |
26.07 |
34.50 |
33.18 |
28.65 |
6.11 |
||
Table 2: Cytotoxic effect of different extracts of the selected fruits.

Figure 1: Dose dependent inhibitory effect of the active extracts of the selected fruits extracts against carcinoma cell lines (A) PPHE (B) GSHE.
To trace the mechanisms by which the most active extracts induce death to cancer cells, we used a staining assay to detect the changes that occurred in the nucleus of treated HCT-116 cells. The cells treated with the pomegranate peel hexane extract (PPHE) and grape seed hexane extract (GSHE) exhibited morphological modifications consistent with apoptosis in a time-dependent manner. After 6 h, the nucleus of cells treated with PPHE and GSHE at (50 µg/mL) started to condense, the chromatin shrunk, and the nuclei became crescent shaped. After 12 h of treatment, many cells had detached chromatin bodies, which are the characteristic sign of late stage apoptosis. In contrast, untreated cells showed intact nuclei with no cellular changes (Figure 2A). The apoptotic indices estimated for GSHE (50 µg/ml) after 6 and 12 h treatment were 18.8 ± 3% and 37.7 ± 2.9% respectively. The apoptotic index for PPHE at 50 µg/ml after 6 and 12 h treatment were 26.4 ± 1.3% and 48.7 ± 2.9% respectively (Figure 2B).
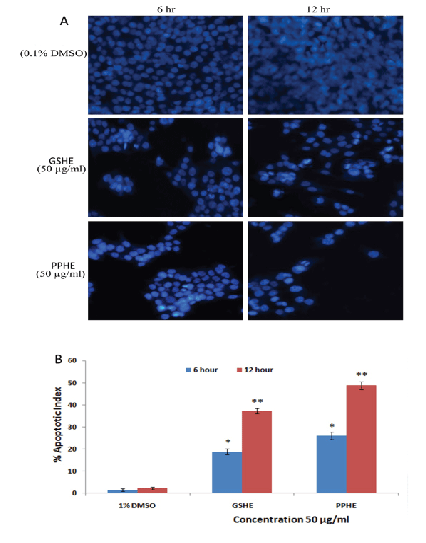
Figure 2: The photomicrographs depict the images of HCT 116 cells with Hoechst 33258 stain, under an inverted phase-contrast microscope at 200x magnification using a digital camera at 6 and 12 h after treatment with GSHE and PPHE. (A) The cells treated with 0.1% DMSO (Vehicle) showed an intact cell membrane with prompt and evenly distributed nucleus in cytosol, whereas Cells treated with 50 µg/ml GSHE and PPHE after 6 h displayed early stage apoptotic such as membrane blebbing and chromatin condensation .Subsequently, the cells treated with treated samples after 12 h revealed late staged apoptotic produced clear singe like nuclear dissolution and crescent shaped apoptotic nuclei. (B) Graphical representation of percentage of apoptotic indices HCT 116 cells. The apoptotic index was expressed as a percentage of the ratio of number of apoptotic cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n=6), (*=p<0.05 and **=p<0.01).
The rat aorta ring assay was used to investigate the antiangiogenic activity of different fruit extracts (Figure 2) shows the rat aorta tissue explants for the ex vivo angiogenesis screening study. Figure 2A shows sprouting of massive microvessels from untreated explanted tissue. Figures 2B-2H showed the effects of the date fruit n-hexane extract, fig ethanol extract, grape seed water extract, grape skin ethanol extract, olive skin ethanol extract, pomegranate peel ethanol extract, and the standard reference suramin, respectively.
Fruit extracts with >60% inhibition of sprouting of blood vessels were considered to be active extracts. Table 3 shows the antiangiogenic effect of fruit extracts determined by the rat aorta ring assay. Out of 18 extracts, 11 extracts exhibited potent inhibitory activity (>60%). The highest inhibition (100%) (P<0.01) were produced by the ethanol extract of pomegranate peel and olive skin and the n-hexane extract of grape skin. All the grape seed extracts caused significant (P<0.05) inhibition of microblood vessels, with water at 84.56% followed by ethanol (76.99%) and n-hexane (67.27%). The n-hexane extract of date fruit also had a significant antiangiogenic effect (87.21% inhibition). These significant inhibition values were very much comparable with the standard drug (suramin) (Table 4), which demonstrated potent inhibition of microvessel growth (Figure 3).
Active extracts |
Carcinoma Cell Lines |
|||
HCT-116 |
MCF-7 |
U-78 MG |
PC-3 |
|
PPHE |
29.31 ± 1.32 |
52.71 ± 2.11 |
59.40 ± 1.58 |
65.23 ± 2.21 |
GSHE |
33.50 ± 2.80 |
49.30 ± 2.61 |
61.18 ± 1.77 |
- |
Table 3: IC50 (µg/ml) values of the active extracts of the selected fruits
The values are expressed as mean ± SEM (n=4). PPHE: Pomegranate peel hexane extract; GSHE: Grape seed hexane extract.
Fruits |
Part used |
Extract |
Inhibition (%) |
Date |
Fruit |
n-hexane |
87.21 ± 2.13* |
Ethanol |
55.46 ± 3.54 |
||
Water |
30.83 ± 2.32 |
||
Fig |
Fruit |
n-hexane |
46.60 ± 4.91 |
Ethanol |
70.88 ± 2.52* |
||
Water |
40.50 ± 2.56 |
||
Grape |
Seed |
n-hexane |
67.27 ± 1.98* |
Ethanol |
76.99 ± 2.10* |
||
Water |
84.56 ± 1.50* |
||
Skin |
n-hexane |
100 ± 1.21** |
|
Ethanol |
79.44 ± 1.45* |
||
Water |
52.12 ± 2.48 |
||
Olive |
Skin |
n-hexane |
82.44 ± 2,51* |
Ethanol |
100 ± 0.85** |
||
Water |
48.52 ± 3.75 |
||
Pomegranate |
Peel |
n-hexane |
67.94 ± 2.41* |
Ethanol |
100 ± 1.12** |
||
Water |
52.72 ± 2.97 |
||
Suramin |
- |
- |
100 ± 0.05** |
Table 4: Antiangiogenic activity of the selected fruit extracts on rat aortic explants
Results are presented as mean percent inhibition ± SEM, (n=6). Statistical significance is expressed as *=P<0.05, **=P<0.01 compared to negative control group (0.1% DMSO).
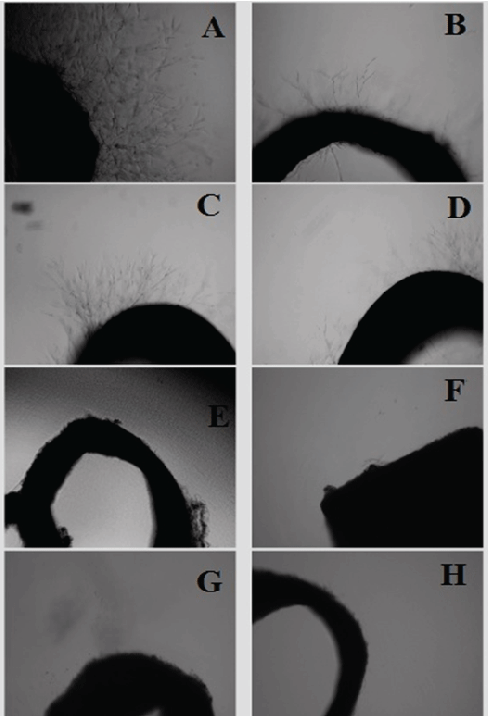
Figure 3: Photomicrographs showing antiangiogenic effect of extracts of selected fruits (100 µg/mL) against sprouting of microvessels in rat aortic explants. A) Negative control showing extensive growth of microvessels. B) Treatment with the date fruit n-hexane extract displaying potent inhibition of growth of microvessels. C) Treatment with fig fruit ethanol extract showing significant inhibitory effect against sprouting of microvessels. D) Treatment with grape seed water extract showing strong antiangiogenic effect. E) Treatment with ethanol extract of grape skin showing a significant inhibitory effect on growth of microvessels. F) Treatment with ethanol extract of olive skin. G) Treatment with pomegranate peel ethanol extract. H) Treatment with standard reference control suramin showing strong inhibitory effect on microvessels growth.
The active extracts were subjected to GC-MS analysis to identify and quantify their major chemical constituents (Figures 4–9). The chemical composition of the fruits extract was identified using the NIST library. The major components present in the extracts are as follows:
With regard to the wide range of phytochemical classes in fruits, phenolic acids, sterols and triterpenoids, fatty acids, triglycerides, and alkaloids appear to be the dominant forms in these fruits.
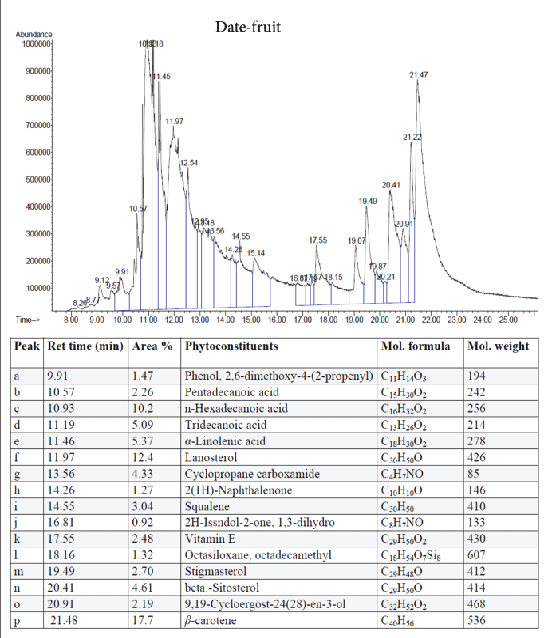
Figure 4: GC-MS quantitative estimation of phytochemicals in hexane extract of dried date fruits.
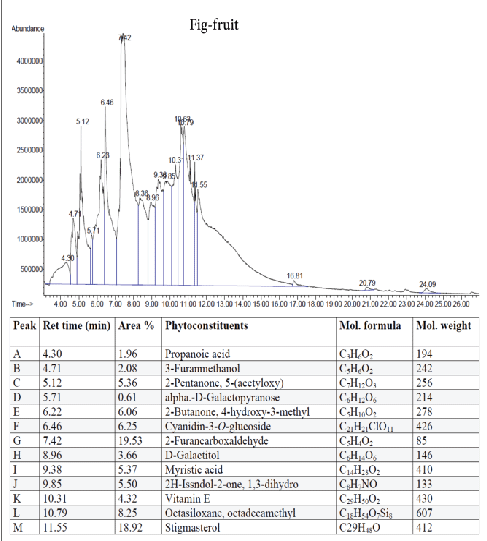
Figure 5: GC-MS quantitative estimation of phytochemicals in ethanol extract of dried fig fruits.
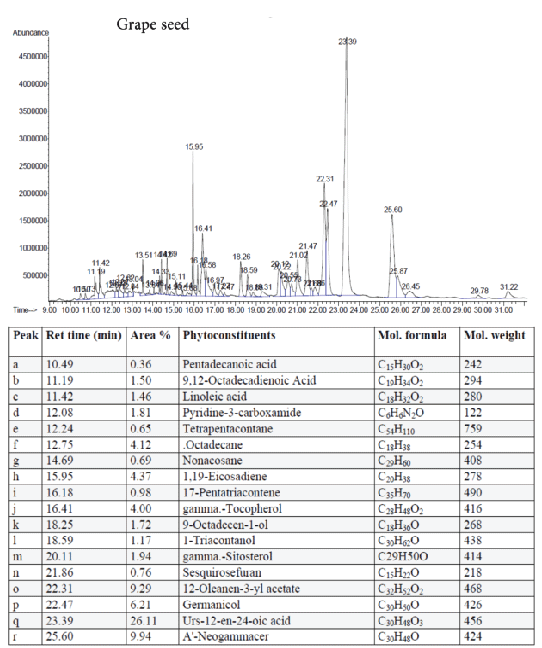
Figure 6: GC-MS quantitative estimation of phytochemicals in hexane extract of dried grape seed
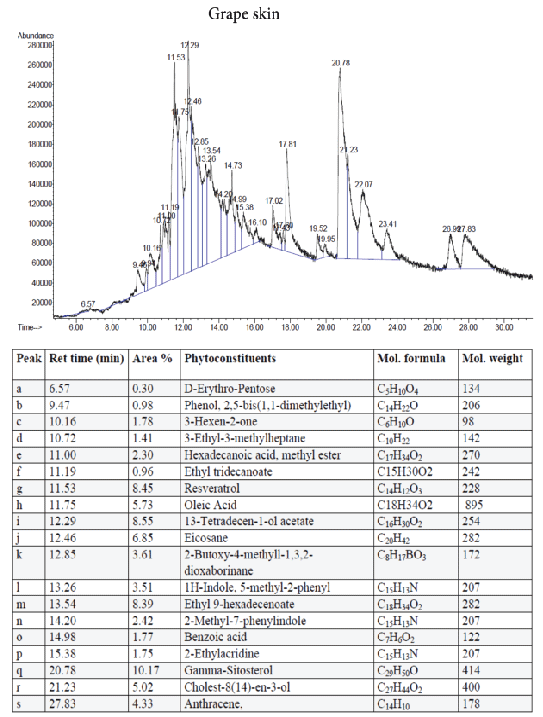
Figure 7: GC-MS quantitative estimation of phytochemicals in hexane extract of dried grape skin.
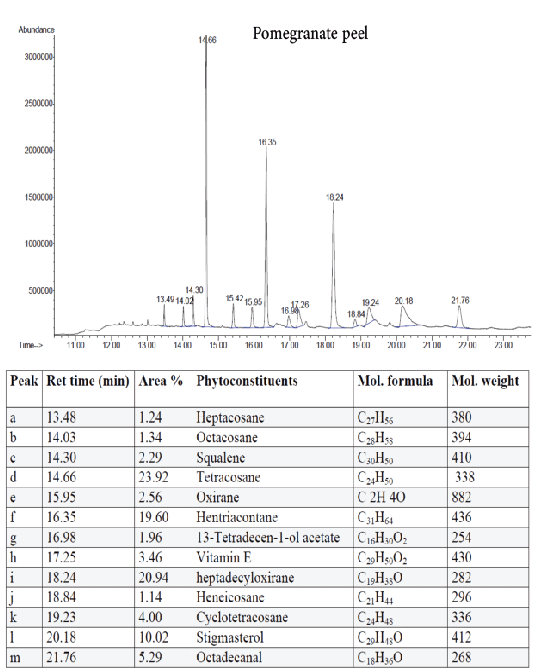
Figure 8: GC-MS quantitative estimation of phytochemicals in hexane extract of dried pomegranate peel.
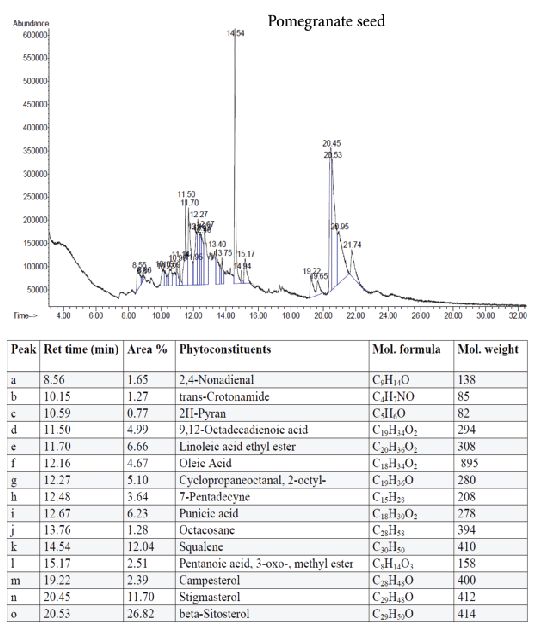
Figure 9: GC-MS quantitative estimation of phytochemicals in ethanol extract of dried pomegranate seed.
Despite the many advances that have been made in cancer therapy, colon, breast, and prostate cancer are among the most frequent cancer types that cause morbidity and mortality worldwide. Although there are many therapeutic approaches to treating cancer, results are not fully satisfactory because the cytotoxicity of chemotherapy to solid tumors is nonspecific. Moreover, selective anticancer drugs are lacking and some recurring tumors can become resistant to drugs [19]. The capacity of a chemotherapeutic agent to target malignant cells while preserving normal cells is the hallmark of a promising cancer drug. Increasing evidence from both experimental and clinical studies suggests that a phytochemical-rich diet may interfere with a specific stage of the carcinogenic process [19,20]. Epidemiological evidence shows that consumption of fruits and vegetable is associated with a lowered risk for developing chronic diseases, such cancer, diabetes, Alzheimer’s disease, and heart disease.
Phytoconstituents found in fruits, including flavonoids, phenolics, and carotenoids, are thought to be responsible for the health benefits of fruits. Many studies have shown that extracts obtained from fruits have strong antioxidant and antiproliferative properties [20]. Thus; they may prove to be an alternative strategy to treating and preventing cancer. In fact, a dietary regime is a practical way to apply a combination of nontoxic, effective phytochemicals from fruit extracts to enhance the efficacy of chemotherapy and lower toxicity to normal cells.
In the present study, different parts of five fruits were extracted sequentially with n-hexane, ethanol, and water to prepare 21 extracts. Two assays were used to assess the antitumorgenesis properties of the fruits extracts. Cytotoxicity of the extracts was measured using the MTT assay, which shows cell viability via mitochondrial activity in living cells. The ex vivo rat aorta ring assay was used to evaluate the effect of fruit extracts on the growth of new blood vessels (angiogenesis) from explanted tissue. The cytotoxicity assay showed that the highly non-polar solvent extract (i.e., n-hexane extracts) had the highest cytotoxic activity among the extracts tested. Among the five fruits, the hexane extract of pomegranate peel had a strong antiproliferative effect with an IC50 values 29.31 ± 1.32, 52.71 ± 2.11, 59.40 ± 1.58 and 65.23 ± 2.21 µg/ml on HCT-116, MCF-7, U-78 MG and PC-3, respectively. Both the non-polar (hexane) and polar (ethanol) grape seed extracts had selective cytotoxicity towards the colon cancer cell line (HCT-116) and the prostate cancer cell line (PC-3). Similarly, date fruit and olive skin hexane extracts had selective effects against the brain cancer cell line (U-78 MG) and the colon cancer cell line (HCT-116), respectively. The grape skin extract exhibited selective cytotoxicity against the breast cancer cell line (MCF-7). In contrast, the fruit extracts were not cytotoxic to the normal cell line (HUVEC).
The most active extract was the pomegranate peel n-hexane extract (PPHE), and it was found to induce apoptosis in HCT-116 cells (Figure 2) through a series of morphological changes, such as membrane blebbing, chromatin condensation, and nuclear fragmentation. However, the results of the antiangiogenic study showed that out of the 21 extracts from the five fruits, only 11 showed a strong inhibitory effect (>60%). In particular, the ethanol extract of pomegranate peel, olive skin and the grape skin hexane extract inhibited 100% of the aortic microvessels. Interestingly, all of the grape seed extracts demonstrated significant antiangiogenic activity. This study also aimed to assess the correlation between antiangiogenic effect and antioxidant activity of different extracts from selected Mediterranean fruits. The ethanol extracts of pomegranate peel and olive skin also exhibited potent antioxidant activity, with IC50 values of 6.06 and 10.11 µg/mL, respectively.
Tumor angiogenesis or tumor vasculature is very crucial for propagation and progression of the tumor invasion and metastasis. It helps to sustain the growth of cancerous cells with oxygen and nutrients. A significant correlation was shown to exist between the degree of tumor angiogenesis and survival rate in patients suffering from solid tumors [21]. Therefore, blood-vessels ablation or prevention could be a good target for cancer therapy. The quantitative study of phytoconstituents in the selected active extracts using GC-MS revealed a wide range of bioactive constituents (Figures 4–9) that have dynamic therapeutic properties (e.g., antioxidant and anticancer). The major form of carotenoids present in date fruit was β-carotene (Figure 4), which has been reported to have antioxidant activity in vitro and in vivo [22,23]. In a recent study, the ethanol extract of the fig reduced the viability of HeLa cells and had an IC50 value of 12 µg/mL [24]. Pharmacological and chemical studies have demonstrated anticancer and anti-inflammatory properties of both crude and isolated compounds from fig fruit [20]. Included in the chemical profile of the fig in the current study was cyanidin-3-O-glucoside (Figure 5). Solomon et al. [25], reported that this compound is the major anthocyanin in fig fruit and that it has high antioxidant capacity. Other research has focused on grape-based products as potential sources of therapeutic and chemopreventive agents against many forms of cancer [26-28]. In the present study, boswellic acid was the main triterpene present in the grape seed extract. Several studies have reported that boswellic acid suppresses the growth and metastasis of human colon and pancreatic cancer by modulation of multiple targets [29,30]. Furthermore, the pomegranate and its bioactive products also have been the subject of many therapeutics applications, including use as anantioxidant, anticarcinogenic, antiinflammatory agent and to treat erectile dysfunction, bacterial infections, and antibiotic resistance [31]. A plethora of evidence indicates that the pomegranate extracts including (seed, peel, juice and whole fruit) are a convenience source for chemopreventive and/or cancer therapeutic effects, demonstrated in several cancer cell lines and animal models [32]. In particular, since the peel fraction of pomegranate is a valuable reservoir of the majority of phenolic compounds, it has been frequently utilized as a natural antioxidant in various dietary supplements [33]. Our results indicated that the hexane extract of pomegranate peel exhibited significant anticancer effects against all tested cancer cell lines, with more selectivity towards human colorectal carcinoma cell line HCT-116 and breast cancer cell line MCF-7 with IC50 value of 29.31 ± 1.32 and 52.71 ± 2.11 µg/mL, respectively. More recently, a study has reported that Iranian pomegranate peel extracts displayed potent antioxidant and anticancer properties, MCF-7 cells were the most responsive cells to cytotoxic effect with a maximum mean growth inhibitory of 81.0% [34]. In general, the biological activities of the pomegranate derivatives are partially attributed to their broad spectrum of vital micronutrients and phytochemicals. The mechanistic aspect of pomegranate extracts may prevent cancer through various mode of action including antioxidant activity, inhibition of cell proliferation, induction of apoptosis and inhibition of angiogenesis process.
In conclusion, the present study revealed that among 21 extracts prepared from selected fruits, the hexane extracts of pomegranate peel, olive skin, and grape were the most antiangiogenic and antiproliferative agents. The biological activities observed in this study can be attributed to the antioxidant activity and the high percentage of bioactive constituents, including β-carotene, beta-sitosterol, squalene, vitamin E, boswellic acid, gamma-sitosterol, oleic acid, linoleic acid, and punicic acid. Thus, the extracts of these fruits are promising candidates for novel chemopreventive or chemotherapeutic formulations to treat various diseases.
The authors declare that there is no conflict of interests.
Download Provisional PDF Here
Article Type: Research Article
Citation: Dahham SS, Tabana YM, Hassan LEA, Ezzat MO, Zulkepli NN, et al. (2016) Antiangiogenic, Antioxidant, and Antiproliferative effects of Common Mediterranean Fruit Extracts with Phytochemical Screening. J Drug Res Dev 2(4): doi http://dx.doi.org/10.16966/2470-1009.121
Copyright: © 2016 Dahham SS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access