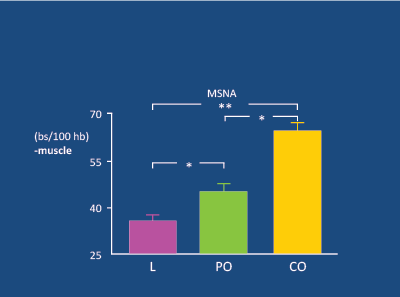
Figure 1: In 30 lean (L), 20 peripherally obese (PO) and 26 centrally obese (CO) subjects (mean age 36y), muscle sympathetic nerve activity (MSNA) was significantly higher in CO than PO and L subjects. Grassi G et al. 2004 [14]


JM Cruickshank*
Oxian Cardiovascular Consultantancy, UK*Corresponding author: JM Cruickshank, BM, BCH, MA (Oxford), DM (Oxford), FRCP (London), Oxian Cardiovascular Consultantancy, UK, E-mail: Johndtl@aol.com
Summary and conclusion
There is an obesity/type-2 diabetes/hypertension epidemic in developed countries around the world. Central obesity is closely linked to hypertension and type-2 diabetes in young/middle-age. In this younger, probably obese, age-group diastolic hypertension is linked to increased sympathetic nerve activity (via raised plasma insulin and leptin levels acting upon the hypothalamic region), particularly in the presence of type-2 diabetes. Chronically raised sympathetic nerve activity and beta-receptor levels (in lymphocytes), independent of blood pressure, are powerful predictors of myocardial infarction in the middle-aged. This has treatment implications for the young/middle-aged hypertensive subjects, with or without type-2 diabetes.
Antihypertensive agents that increase sympathetic nerve activity e.g. dihydropyridine calcium blockers, thiazide-type diuretics, and angiotensin receptor blockers, do not reduce (and may increase) the risk of myocardial infarction in the younger/middle-aged hypertensive subject. Beta- 1 blockade, effective in reversing and stabilizing coronary atheromataous plaque, is at least as good as ACE-inhibition in preventing hard cardiovascular endpoints (including myocardial infarction, and is significantly superior in preventing all-cause death). Thus, beta-1 blockade is a highly reasonable first-line treatment in young/middle-aged hypertension with or without type-2 diabetes.
Diabetes; Hypertension; Sympathetic nerve activity
BP: Blood Pressure; HT: Hypertension; DM2: type-2 diabetes; BB: Beta-blocker; ARB: Angiotensin Receptor Blocker; SNA: Sympathetic Nerve Activity; BMI: Body Mass Index; MI: Myocardial Infarction.
In the last 30 years or so there has been a global increase in obesity [1], being particularly apparent in the USA [1,2] the UK and Australia [3,4]. Obesity tends to be less prevalent among educated wealthy individuals [2].
Weight-gain is associated with an increased risk of type-2 diabetes (DM2) [5]. DM2 is associated with a two-fold increase in cardiovascular events [6]. Weight-gain, in addition to other life-style factors such as physical inactivity, a Westernized diet and alcohol-abuse, is also responsible for about 70% of cases of essential hypertension (HT) in younger/middle-aged subjects [7,8].
This mini-review sets out to examine the inter-relationships between obesity, DM2, and HT, and to highlight the importance of raised sympathetic nerve activity (SNA) and treatment implications.
The classic Framingham Study has investigated the origins of essential HT in a normal (primarily white) population [9] (Table 1). It is apparent that (1) The development of diastolic (± systolic) HT is closely associated to a younger age and an increased body mass index (BMI), and (2) The development of isolated systolic HT occurs in an older age-group, reflecting stiffening/aging of the arteries. Essential HT in the younger agegroup is linked primarily to an increased cardiac output [10], while in the elderly (say greater than 60 years old), where there is a fall in cardiac output [11,12], high BP is dependent upon an increased peripheral resistance.
Predictors of Diastolic Hypertension (± Systolic |
Predictors of Isolated Systolic Hypertension=SBP ≥ 140 mmHg+DBP<90 mmHg |
1. Young age |
1. Older age |
2. Male sex |
2. Female sex |
3. High BMI at baseline |
3. Increased BMI during follow-up (weak) |
4. Increased BMI during follow-up |
4. ISH arises more commonly from normal and high normal BP, than “burned out” diastolic hypertension |
5. Main mechanism of DH and SDH is raised peripheral resistance |
5. Only 18% with new – onset ISH had a previous DBP ≥ 95 mmHg |
6. Main mechanism of ISH is increased arterial stiffness=aging of arteries |
Table 1: Different Predictors of Diastolic Hypertension (DH) (± raised systolic–SDH) and Isolated Systolic Hypertension (ISH)–FRAMINGHAM Study [9]
Obese adolescents with HT experience a substantial fall in BP after weight-loss following bariatric surgery, with 74% becoming normotensive [13]. In younger subjects, obesity (particularly central) is linked to a significant increase in SNA in muscle [14] (Figure 1). In men, there is a powerful linear relationship between waist circumference and SNA [15] (Figure 2). Obesity-related increases in SNA are particularly apparent in the presence of HT [16], especially if DM2 is also present [17] (Figure 3). The raised SNA is associated with the release of leptin (so-called “thin hormone”) from central adipose tissue; leptin acts upon the hypothalamic region of the mid-brain, resulting in increased SNA [18]. High insulin levels, associated with obesity-related insulin resistance, also act upon the hypothalamic region, resulting in heightened SNA [19,20].

Figure 1: In 30 lean (L), 20 peripherally obese (PO) and 26 centrally obese (CO) subjects (mean age 36y), muscle sympathetic nerve activity (MSNA) was significantly higher in CO than PO and L subjects. Grassi G et al. 2004 [14]
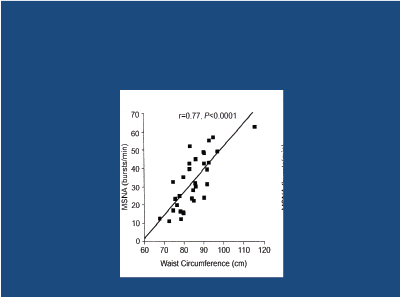
Figure 2: Relationship between waist-circumference and muscle sympathetic nerve activity (MSNA) in men.
Joyner MJ et al. 2010 [15]
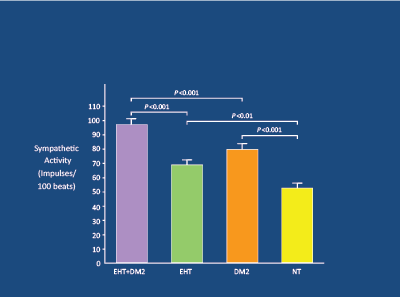
Figure 3: In 68 matched subjects (n=17-NT; 17-DM2; 17 HT; 17 DM2+HT),
sympathetic activity markedly raised in DM2+HT, and correlated with high insulin levels. Huggett RJ et al. 2003 [17]
High levels of SNA are associated with a poor long-term prognosis. Firstly, high norepinephrine (noradrenaline) levels are associated with the atherosclerotic process [21] and (via an increased heart rate) coronary plaque rupture [22]. Secondly, high plasma norepinephrine levels, independent of smoking and blood pressure levels, are powerful predictors of cardiovascular death and survival in young/middle-aged hypertensive subjects over a 6-7 year follow-up period [23] ( Figure 4). Importantly, high intra-lymphocyte beta-receptor density (Bmax) and cyclic adenosine monophosphate (DMP) levels predict (independent of BP) future myocardial infarctions, but not stroke (which relates to BP) [23] (Figure 5).
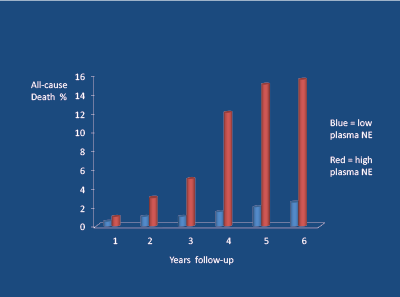
Figure 4: 601 middle-aged hypertensive subjects followed-up for 6-7 years; high plasma norepinephrine concentrations (NE) (> 4.0 nmol/L = red) vs low (> 4.0 nmol/L=blue) were associated with high levels of all- cause death (independent of blood pressure). Peng Y-X et al 2006 [23]
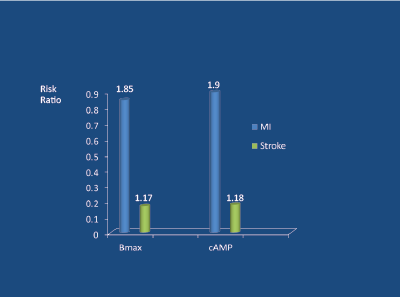
Figure 5: Beta-receptor density (Bmax) and cAMP levels (in lymphocytes) as predictors of MI and stroke in middle-aged hypertensives followed for 7 years. Peng Y-X et al 2006 [23]
High resting heart rates are a surrogate for increased SNA. The Framingham Study [24] has shown that in young/middle-aged hypertensive subjects, high resting heart rates (particularly over 85 bpm) have been shown to predict all-cause death and cardiovascular and coronary heart disease events for both hypertensive men- (Figure 6) and women, over a 36 year follow-up period.
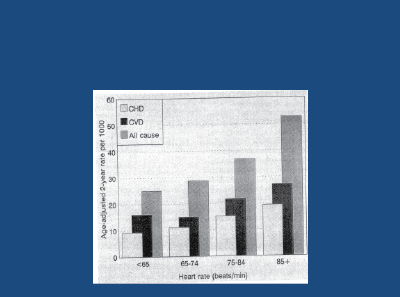
Figure 6: Framingham: Effect of resting heart rate on all-cause death, CHD and CVD events in untreated male hypertensives, followed-up for 36 years. Gillman MW et al. 1993 [24]
Beta-1 stimulation of the renal juxtaglomerular apparatus results in the release of renin. Thus, high plasma renin activity (PRA), like high sympathetic activity [23], could be an indicator of a poor prognosis. It is note-worthy that in high PRA cases, beta-blockers have a particularly powerful anti-hypertensive effect [25].
Antihypertensive agents that increase SNA have performed poorly in terms of reducing cardiovascular events in young/middle-aged hypertensive subjects.
Thiazide-type diuretics increase SNA [26], and in 3 studies involving diuretic therapy in young/middle-aged hypertensive subjects [27-29] there was no reduction in the risk of myocardial infarction, and even a significant increase [29], versus randomised placebo/non-treatment.
Dihydropyrididne calcium blockers increase heart-rate and plasma norepinephrine levels [30], and in the ABCD study involving middle-aged hypertensive subjects with DM2 [31], the investigation was terminated prematurely due to a significant excess of myocardial infarctions in the nisoldipine, vs the enalapril, group.
Angiotensin receptor blockers (ARBs) increase SNA in younger subjects [32,33]. Meta-analyses indicate that, in contrast to ACE-inhibitors, ARBs increase the risk of myocardial infarction [34,35] (Figure 7). In two subsequent placebo-controlled studies involving hypertension [36] and pre-hypertension plus DM2 [37] (Figure 8), there was a significant excess of fatal cardiovascular events in those receiving the ARB.
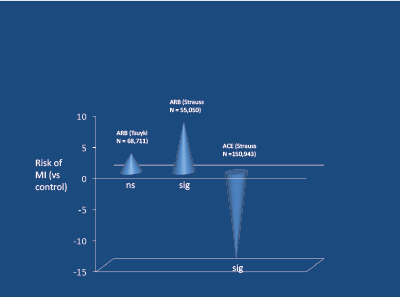
Figure 7: Relative risk of MI in meta-analyses of ARB and ACE-inhibitors. Strauss and Hall, Circulat 2007 [35]
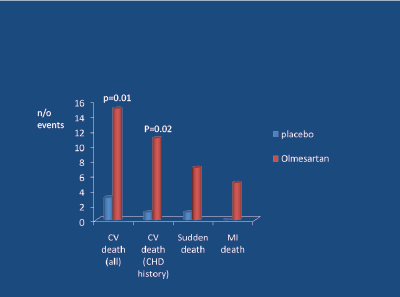
Figure 8: ROADMAP Study; Olmesartan vs placebo (randomised) in 4447 DM2, mean age 57, mean BMI 31, BP 136/81, over 3.2 years. Haller H et al. NEJM 2011 [37]
ACE inhibitors reduce SNA [38], and performed well vs the calcium blocker nisoldipine in terms of fewer myocardial infarctions [31]. In the classic UKPDS-39 study [39], involving middle-aged hypertensive subjects with DM2, atenolol was compared to captopril in terms of reduction of 7 primary endpoints versus less-tight control (10/5 mg Hg) of BP, over a 9 year period of observation. The effects of the 2 agents upon the 7 primary endpoints (plus heart failure, a secondary endpoint), vs less-tight control, are shown in figure 9. It is apparent that all 8 trends favoured atenolol (over captopril). Compared to less-tight control of BP, atenolol reduced stroke-risk by about 50%, peripheral arterial disease-related endpoints by about 60%, micro-vascular (kidney and eye) endpoints by about 45%, and heart failure by about 65%. After 14.5 years long-term follow-up, the above trends persisted but now there was a significant (23%) reduction in all-cause death in favour of atenolol [40] (Figure 10).
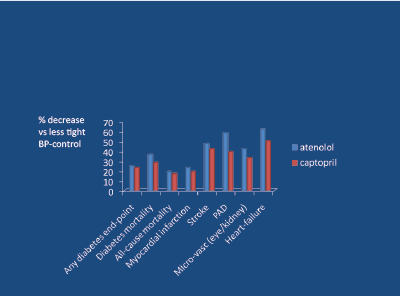
Figure 9: UKPDS 39 – all primary end-point trends favour atenolol vs captopril when compared with less-tight BP control (BP diff 10/5 mm Hg)
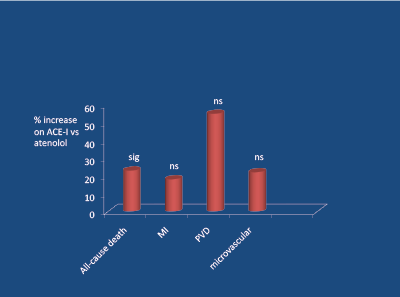
Figure 10: UKPDS study 20 year follow-up (mean 14.5 years); significant (p<0.05) increase in all-cause death on ACE-I (vs atenolol). Holman RR et al. 2008 [40]
Recent negative messages regarding the role of beta-blockers (BBs) in the treatment of HT, have arisen from meta-analyses that did not take age into account [6,41-47]. Two meta-analyses that did take age into account arrived at very different conclusions [46,47]. Compared to randomised placebo, in the younger hypertensive subject (mean age less than 60 years old) BBs were significantly superior to randomised placebo in reducing the risk of death/stroke/MI (Figure 11), with only a positive trend in the elderly. When compared to randomised comparator antihypertensive agents, BBs were at least as effective as randomised comparator drugs in reducing the risk of death/stroke/MI in younger subjects (Figure 12), in contrast to those older than 60 years old, where BBs were significantly less effective in reducing the risk of death/stroke/MI. Thus, BBs are an effective first-line option regarding the treatment of younger/middle-aged (less than 60 years old) hypertensive subject. In the elderly hypertensive subject, first-line BBs are appropriate only if myocardial ischaemia is also present [48].
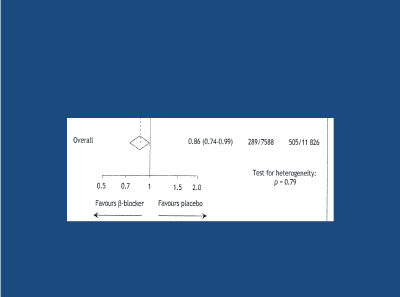
Figure 11: A meta-analysis of 2 studies in the younger (< 60y) hypertensive subject; beta-blockers significantly superior to randomised placebo in preventing all cause death/stroke/MI. Khan and McAlister, 2006 [46]
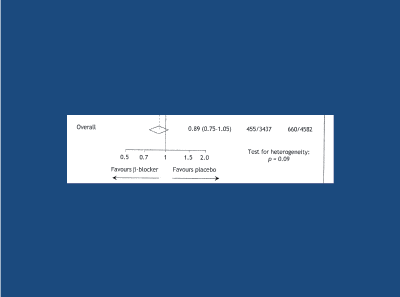
Figure 12: Meta-analysis of 5 studies in the elderly hypertensive subject (> 60y) – a strong trend favouring beta-blockers vs randomised placebo in the prevention of the composite death/stroke/MI. Khan and McAlister 2006 [46]
In 3 major prospective, randomised, hard-endpoint studies in middleaged hypertensive subjects, cigarette smoking played a key role in modifying the potential of the BB to reduce the risk of a cardiovascular event. The MRC-1 study [28] compared non-selective propranolol with a thiazide diuretic and placebo; the IPPPSH study [49] compared nonselective oxprenolol with placebo; the MAPHY [50] compared moderately beta-1 selective metoprolol with a thiazide diuretic.
In the case MI (about 3 times more common than stroke in the younger subject), the ability of the BB to reduce the risk of an event by 33-49% (versus randomised placebo or diuretic) in non-smokers, was not observed in smokers [28,49,50]. Indeed, in the case of non-selective propranolol and oxprenolol, the risk of MI was actually increased by 13- 35% in smokers (Figure 13). A similar result relating to stroke was noted in the MRC-1 study [28].
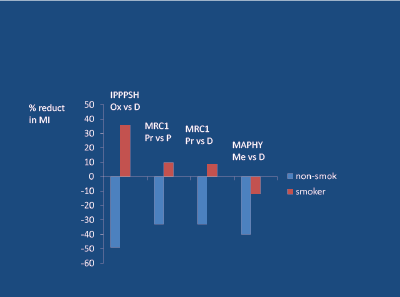
Figure 13: Beta Blocker/Smoking interaction in young/mid-age hypertensives, regarding myocardial infarction (MI); Ox=Oxprenolol, Pr=Propranolol, Me=Metoprolol, P=Placebo, D=Diuretic.
How can these events relating to smokers be explained and avoided? Cigarette smoking is linked to a two-to-threefold increase in plasma epinephrine (adrenaline) levels [51]. Epinephrine stimulates beta-1, beta-2 and alpha-receptors, and in the presence of non-selective BBs (and to a lesser extent with only moderately beta-selective agents like metoprolol and atenolol) there is unopposed (total or partial) alphavasoconstriction, resulting in an increase in BP [52]. This increase in BP is about 30 mm Hg for non-selective BBs and about 9-10 mm Hg for a moderately selective agent like metoprolol, compared to no change in BP (vs control) with a highly beta-1 selective agent like bisoprolol (which permits full beta-2 stimulation-induced vasodilatation [53,54] (Figure 14).
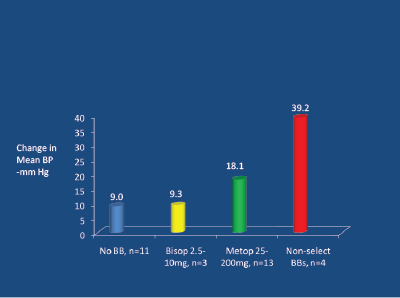
Figure 14: Peri-operative interaction between adrenaline and beta-blockers. Tarnow J and Muller R, 1991 [52]
a) Pharmacokinetic properties
These properties have been described [55] (Table 2). As a general rule -
b) Pharmacodynamic properties
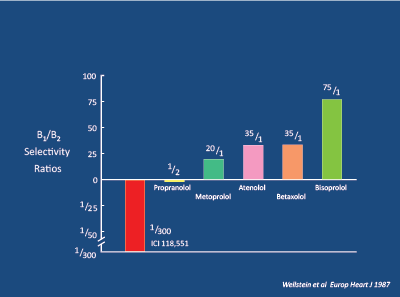
Figure 15: Beta and Beta Selectivity Ratios. Wellstein A et al. 1986 [53]
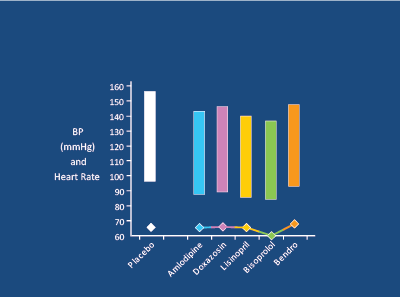
Figure 16: In 34 young (28-55yrs) hypertensives, Bisoprolol 5mg was more effective than Amlodipine 5mg, Doxazosin 104mg, Bendrofluazide 2.5mg, Lisinopril 2.5-10mg (double blind, crossover, 1 month each) in controlling office and 24 hr BP. Deary AJ et al. J Hypertens 2002 [74]

Table 2: Pharmacokinetics of commonly used beta-blockers
From: Cruickshank JM, Prichard BNC (1994) Beta-blockers in Clinical Practice. 2nd Edition. Edinburgh: Churchill Livingstone, 1119-1126 [28].
Download Provisional PDF Here
Article Type: Mini Review
Citation: Cruickshank JM (2016) Diabetes and the Younger/Middle-Aged Hypertensive Subject; Obesity, Sympathetic Nerve Activity and Treatment Implications. J Dia Res Ther 3(1): doi http://dx.doi. org/10.16966/2380-5544.123
Copyright: © 2016 Cruickshank JM. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access