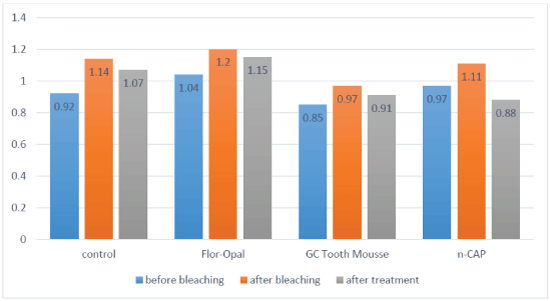
Figure 1: Surface roughness of all study groups before and after bleaching then after treatment.


Ahoud Al-Shamrani1 Wedad Awliya2*
1Lecturer, Department of Restorative Dental Sciences, King Saud University, Kingdom of Saudi Arabia*Corresponding author: Wedad Awliya, Professor, Department of Restorative Dental Sciences, King Saud University, Riyadh, Kingdom of Saudi Arabia, Tel: +966505294516; E-mail: wawliya@hotmail.com
Purpose: To investigate the effect of neutral sodium fluoride gel (Flor-Opal), Casein phosphopeptide-amorphous Calcium Phosphate is containing paste (GC Tooth Mousse) and nano-carbonate Apatite Paste (n-CAP), on enamel surface roughness and staining ability after home bleaching in vitro.
Material and Methods: Eighty enamel specimens were divided into four groups according to enamel surface treatments that were applied after bleaching (n=20). Group 1 (control group) kept in artificial saliva, group 2 assigned to Flor-Opal gel, group 3 for GC Tooth Mousse and group 4 for n-CAP. Baseline average roughness (Ra1 ) of all surfaces was measured using Talysurf Intra 50 instrument profilometer, and the initial color of the specimens (ΔE) was recorded using Color Eye 7000A Spectrophotometer. The specimens were subjected to bleaching for 2 weeks with 10% carbamide peroxide. After bleaching, the average surface roughness (Ra2) and the color difference (ΔE1) measurements were retaken. Then, surface treatments were applied for 2 weeks according to manufacturer instructions for the four groups then the third average surface roughness (Ra3) was recorded. After that, specimens were stained with coffee 10 minutes per day for 2 weeks. Finally, measurements of color differences after staining were evaluated (ΔE2).
Results: Statistically significant increase in surface roughness and color change of all enamel specimens were noticed after bleaching. After the application of the three surface treatments, enamel treated with n-CAP showed a significant reduction in surface roughness (P=0.002) and recorded the lowest color change after staining (ΔE2 =4.1). The treatment of bleached enamel surfaces with Flor-Opal and GC Tooth Mousse did not significantly reduce the increased roughness resulted from bleaching (p=0.57 and 0.08 respectively). However, no significant difference in color was found between the specimens treated with Flor-Opal, GC Tooth Mousse, and specimens treated with n-CAP after staining.
Conclusions: N-CAP was effective in reducing enamel surface roughness resulted from bleaching. Also, n-CAP was able to reduce enamel re-staining after bleaching.
Home bleaching; Enamel surface roughness; Color; Staining; Fluoride; Amorphous calcium phosphate; Nanocarbonate
Teeth color is one of the reasons for seeking dental esthetic treatment [1]. It has been proven that self-satisfaction with the teeth color decreases with increasing severity of discoloration [2]. Dentist can treat discolored teeth with a variety of methods ranging from removal of surface stains, bleaching, veneers and crowns depending on the severity of discoloration. There are several methods to whiten teeth such as the use of bleaching agents [3,4]. Hydrogen peroxide, carbamide peroxide or sodium perborate are the main active chemical components of most agents used in teeth bleaching [5,6].
Peroxides are considered to be safe [7,8]. However, studies have shown changes in chemical composition, mineral content, [9] and surface roughness of bleached teeth [10]. Rough enamel surface can be discolored easily after bleaching [11].
One of the methods to recover enamel defects after bleaching is fluoride treatment. It was found that 0.05% Sodium Fluoride (NaF) applied daily and 0.2% NaF applied weekly was effective in reducing the increase enamel surface roughness after bleaching [12].
Casein Phosphopeptide (CPP) is milk derived protein able to bind calcium and phosphate ions and stabilize them as Amorphous Calcium Phosphate (ACP) [13]. The GC Tooth Mousse based on the Recaldent TM technology is a water-based and sugar-free cream containing 10% CPP-ACP. It is applied directly to the tooth surface to prevent demineralization and promote remineralization [14-16]. Also, it has been found that the application of GC Tooth Mousse after bleaching was effective in preventing the re-staining of bleached teeth [17].
Hydroxyapatite (HAP) materials have been used also to remineralize altered enamel surface. It is one of the most biocompatible and bioactive methods, because of its similarity to the mineral compositions found in teeth [18] if the particle size of hydroxyapatite is reduced to the nano-size, the remineralization effect will be increased [19]. HAP is widely used as an osteoconductive biomaterial by adding carbonate which called carbonate apatite [CAP, Ca10(PO4.CO3)6(OH)2]. Nanocarbonate (n-CAP) bonds chemically to the surface of enamel, therefore it maintains the initial color of bleached teeth and protects the damaged enamel structure after bleaching [20].
The purpose of this study was to investigate the effect of fluoride gels (Flor-Opal), casein phosphopeptide-amorphous calcium phosphate containing paste (GC Tooth Mousse) and nano-carbonate apatite (n-CAP) on enamel surface roughness and staining after home bleaching in vitro.
This study was supported and approved by College of Dentistry Research center and Deanship of Scientific Research, King Saud University/ Saudi Arabia. Research project NF #2358.
Forty human premolars extracted for periodontal or orthodontic reasons were used in the study. All teeth were cleaned with an ultrasonic scaler and polished with non-fluoridated pumice, using a rubber cup mounted in a slow speed hand piece (Kavo EWL, No. 6412500, West Germany), to remove any surface debris or contaminates. After cleaning the teeth, they were stored in distilled water with 0.05% thymol solution. The teeth were examined under a stereomicroscope to exclude the teeth with any caries lesions, structural enamel defects or cracks and previous restorations. The roots of all teeth were removed from the crowns approximately 1 mm below the cemento-enamel junction using a slow-speed diamond saw, (Isomet 2000, Buehler, Lake Buff, Illinois, USA), under water-coolant spray. The pulp chambers were cleaned and further sectioning was made in the mesio-distal direction. Both the buccal and lingual surfaces of the teeth were used for bleaching to give a total of eighty specimens (n=80). Each tooth surface was then embedded in acrylic resin so that the buccal and lingual surfaces face upwards. Then the teeth were randomly divided into four groups.
Before the bleaching with 10% carbamide peroxide, base-line surface roughness (Ra1 ) using a profilometer, (Talysurf Intra 50 instrument, Tylor Hobson Ltd., 112/3477-02, series no.339, Leicester, England), and initial color (∆E) of the specimens using Color Eye 7000 spectrophotometer (Gretag Macbetch, New Windsor, NY, USA) were evaluated. The specimens were then subjected to 14 successive days of 8 hours bleaching with intermittent storages in artificial saliva. At the end of each bleaching session, the bleaching material was removed using suction, and then the specimens were rinsed with tap water. This is to simulate the wearing of night guard for home bleaching and to get the benefit of saliva storage in remineralization of the bleached specimens. After finishing all the bleaching sessions, evaluations of the specimens were done including surface roughness (Ra2) and the color (∆E1). Then, the changes in color were calculated according to the following formula:
• ∆E1 =[(L*1-L*0)2+(a*1-a*0)2+(b*1-b*0)2]1/2
• L*0, a*0, b* 0=Color of the specimens before bleaching
• L*1, a*1, b*1=Color of the specimens after bleaching
• L* represents the value of the color
• a* is the measurement along the red-green axis
• b* is the measurement along the yellow-blue axis
After the bleaching, the treatments were applied for 2 weeks on enamel surfaces of each group; Group1: specimens were covered with 1 mm thickness of For-Opal gel (1.1% neutral sodium fluoride) for two hours according to manufacturer instructions, Group 2: specimens were covered with 1 mm thickness of GC Tooth Mousse (CPP-ACP) for four minutes according to manufacturer instructions and Group 3: specimens were covered with 1 mm thickness of n-CAP paste for four minutes according to Kim and others [20]. Group 4 (control) specimens were kept in artificial saliva for two weeks; Compositions of each material used in this study are presented in table 1. Then the third surface roughness reading (Ra3) was recorded for each specimen. The specimens of all four groups, after that, were immersed in coffee solution 10 minutes per day for 2 weeks. The coffee was prepared according to manufacturer suggested concentration (Table 1). The coffee was allowed to cool to a mean temperature of 50°C.
| Material | Manufacturer | Material Composition | Manufacturer’s instructions |
| Opalescence PF 10% | Ultradent Products Inc, South Jordan, UT, USA | 10% carbamide peroxide, potassium nitrate, carbopol, glycerin, 0.11% fluoride ion, flavoring | Home bleaching gel for 8 h/d for 14 consecutive days |
| Flor-Opal gel (1.1% neutral NaF) | Ultradent Products Inc, South Jordan, UT, USA | 1.1% Neutral sodium fluoride, 2.1% Sodium hydroxide | Applied for 2 hours after bleaching |
| GC Tooth Mousse | GC Corporation, Tokyo, Japan | 10% Recaldent CPP-ACP, glycerol, D-glucitol, Propylene glycol, Colloidal Silica, Sodium carboxyl methyl cellulose (CMC-Na), titanium dioxide, xylitol, Guar Gum, phosphoric acid, Sodium saccharin, zinc oxide, magnesium oxide, ethyl 4-hydroxybenzoate, propyl 4-hydroxybenzoate, Butyl parahydroxybenzoate, Flavor, Distilled water | Direct applications on tooth for 4 minutes |
| n-CAP | was prepared in college of Pharmacy/ King Saud University using previously reported methods [21,22] | Calcium chloride, sodium carbonate double distilled water, Sodium hydroxide, phosphate calcium phosphate, calcium carbonate propylene glycol, glycerol | Applied on tooth for four minutes |
| Coffee | Nescafe Red Mug, Nestle, Zorite 929-Araras/Sp, Brazil | 1 teaspoon of coffee dissolved in 240 ml of boiling water | Specimens immersed in coffee for 10 minutes per day for 2 weeks |
Table 1: Composition of the materials used in the study
The staining ability of the bleached specimens (∆E2 ) was recorded for each group as follow:
• ∆E2 =[(L*2-L*1)2+(a*2-a*1)2+(b*2-b*1)2]1/2
• L*1, a*1, b*1=Color of the specimens after bleaching
• L*2, a*2, b*2=Color of the specimens after staining
The analysis was carried out by using SPSS version 21.0 statistical software. Descriptive statistics (mean and standard deviation) were used to describe the quantitative variables. Student’s one sample t-test was used to compare the material ∆E1 value with the clinically perceivable value (∆E=3.3) as in most studies. One-way analysis of variance was used to compare the mean values of ∆E2 of the four materials followed by tukey’s test for multiple comparisons. Student’s pair t-test also was used to compare the pair mean values of (i) surface roughness of before and after bleaching, (ii) surface roughness after bleaching and after treatment application and (iii) surface roughness after treatment with the baseline roughness for each of the 4 materials. A p-value of ≤ 0.05 and 95% confidence intervals for the difference of mean was used to indicate the statistical significance and precision of the estimates.
Table 2 presents the mean surface roughness (in μm) for the samples, before bleaching (Ra1), after bleaching (Ra2), and after applying the different treatments on the bleached enamel (Ra3). Comparing the mean surface roughness values for each assigned group of specimens before and after bleaching, T-test showed a significant increase in surface roughness of all enamel specimens after bleaching as shown in the table 2. T-test, also showed a significant reduction in surface roughness of the bleached enamel after treatment with n-CAP in comparison to the same group following bleaching only. N-CAP was able to retain the enamel surface roughness to baseline (before bleaching) since no significant difference was found between surface roughness values after treatment with n-CAP and before bleaching. The enamel surfaces became smoother than the surfaces before bleaching.
| Material | Stage | Mean (SD) roughness | Difference | Difference | ||
| (Ra2-Ra1) and p-value | (Ra3-Ra2) and p-value | |||||
| Control | Before bleaching (R1) | 0.92(0.26) | 0.22 | 0.05* | 0.07 | 0.16 |
| After Bleaching (R2) | 1.14(0.58) | |||||
| After treatment (R3) | 1.07(0.42) | |||||
| Flor-Opal | Before bleaching | 1.04(0.68) | 0.16 | 0.04* | -0.05 | 0.57 |
| After Bleaching | 1.20(0.56) | |||||
| After treatment | 1.15(0.69) | |||||
| GC Tooth Mousse | Before bleaching | 0.85(0.32) | 0.12 | 0.007* | -0.06 | 0.08 |
| After Bleaching | 0.97(0.30) | |||||
| After treatment | 0.91(0.24) | |||||
| n-CAP | Before bleaching | 0.97(0.34) | 0.14 | 0.004* | -0.28 | 0.002* |
| After Bleaching | 1.11(0.37) | |||||
| After treatment | 0.88(0.40) | |||||
Table 2: Comparing mean values of roughness (μm) of the four groups (control, Flor-Opal, GC Tooth Mousse and n-CAP) before bleaching (Ra1), after bleaching (Ra2) and after surface treatments (Ra3).
*Indicate statistically significant
Although treatment of bleached enamel with Flor-Opal and GC Tooth Mousse did not significantly reduce the increased roughness as a result of bleaching (Table 2), no significant difference in surface roughness was found between bleached enamel treated by those two materials and baseline surface roughness of each group. Figure 1 compares the surface roughness of all groups during the study.

Figure 1: Surface roughness of all study groups before and after bleaching then after treatment.
Figure 2 presents the means of color change of the enamel specimens assigned
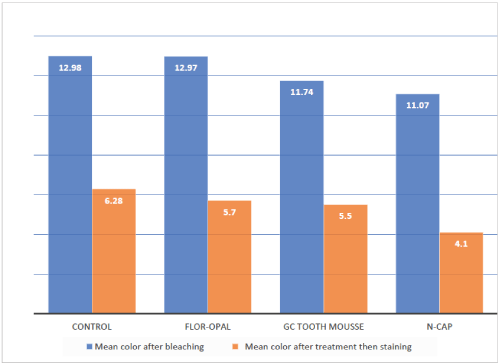
Figure 2: Color change of the study groups after bleaching then after treatment and staining.
to the four test groups after bleaching and after treatments and staining. T-test showed a significant difference between the mean values of ΔE1 of the four groups and the clinically perceivable value 3.3 (p<0.0001). This indicates that the color of all enamel specimens was significantly changed by the bleaching agent. One way analysis of variance (ANOVA) showed a significant difference among the four groups after treatment (p=0.05). Application of n-CAP on enamel surfaces after bleaching resulted in less staining than the no treatment regimen. Tukey’s multiple comparison test showed that ΔE2 of the enamel specimens treated with n-CAP was significantly lower than the control group (p=0.036). However, no significant difference was found between the specimens treated with Flor-Opal and GC Tooth Mousse and the control group (p=0.885 and 0.748 respectively). Although ΔE2 of the enamel specimens treated with n-CAP was lower than those treated with Flor-Opal and GC Tooth Mousse, no significant difference was found between the n-CAP group and the other two groups (p=0.188 with the Flor-Opal and p=0.305 with Tooth Mousse).
The color stability of bleached teeth is influenced by patient dietary habits. Enamel re-staining after bleaching increases if the patients consume more colorants food or drinks such as; coffee, tea or soft drinks [23]. The bleaching agents might cause irregularities, porosities and erosion of enamel surfaces and these changes will make the enamel more prone to re-staining [6]. Therefore, surface treatment applied to freshly bleached teeth could minimize the enamel surface alteration, reduce the absorption of stains and maintain the effect of bleaching for a longer time [11,24].
Although some studies did not find any change in enamel surface roughness after bleaching [25-27], in this study the enamel surface roughness values increased after bleaching. This result is in agreement with the results of other studies, which also found an increase in surface roughness after the application of 10% carbamide peroxide [12,28,29]. This roughness might result from minerals loss due to pH, concentration, frequency and the exposure time to bleaching agent [30]. The bleaching pH (pH~6.5) might be also the cause even it is close to neutral pH. It has been found that bleaching agents’ pH close to 7.0 can cause surface alteration similar to pH values lower than 7.0 [30-32]. Also, the carbopol which is added to the bleaching agent component as a thickener agent may be responsible for changes in enamel mineral content and could cause demineralization of enamel surfaces [33].
The results of the present study showed a decrease in surface roughness after the application of the surface treatments but the reductions were not significant except for n-CAP group. This could be due to the composition of n-CAP which has a more similar inorganic component to the teeth (CAP, Ca10(PO4.CO3)6 (OH)2). The n-CAP has high solubility that allows it to dissolve and redeposit the calcium and phosphate ions in the damaged areas resulted from the demineralization of the bleaching gel [20,34,35].
There was a decrease in the surface roughness values after treatment of bleached enamel with Flor-Opal gel, but the reduction was not statistically significant. This result is in agreement with another study [12]. Fluoride application enhances remineralization by increasing the availability of fluoride ions for the formation of fluoroapatite in the presence of calcium and phosphate ions. Therefore, the benefit of topical application of fluoride treatment will be limited by the availability of calcium and phosphate ions [36].
GC Tooth Mousse can be used as enamel remineralizing agents such as; caries prevention, after bleaching, after scaling and to prevent white spot lesions [13]. The previous study found that the application of CPP-ACP containing paste as post-bleaching treatment reduced significantly the roughness of enamel surface [36]. The free calcium and phosphate ions will move out from the CPP-ACP containing paste when it applied to the tooth. The ions will enter the enamel and replace mineral loss resulted from bleaching [36]. The application of GC Tooth Mousse, in this study, showed a decrease in surface roughness of bleached enamel surfaces, however, this roughness was not statistically significant from the roughness resulted from bleaching.
The general population can distinguish color differences value when ΔE>3.3, which is considered clinically perceivable, and it is used in most of color differences studies in dentistry [2]. All the specimens in this study showed ΔE1 values (color differences of specimens before and after bleaching) above 11.0. This indicates that the bleaching agent was effective in whitening the specimens [37].
The application of n-CAP on bleached enamel surface before staining resulted in significantly less staining than the control group (no treatment regimen). This might be due to the composition of n-CAP as previously mentioned [20,34,35].
Several studies reported that the application of CPP-ACP and fluoride after bleaching was effective in reducing the re-staining of teeth after bleaching [15,18]. However, the results of the present study showed no significant difference between the specimens treated with Flor-Opal or GC Tooth Mousse and the control group after staining. This is also in agreement with another study [38]. On the other hand, no significant difference in color was found between the specimens treated with Flor-Opal and GC Tooth Mousse and n-CAP group. This might indicate that Flor-Opal and GC Tooth Mousse are promising in enamel stain prevention following bleaching.
Finally, further studies are needed to evaluate the microscopic changes after using n-CAP on the surface of human dental enamel. Also, to investigate other clinical applications of the material that can help in reducing the adverse effect of bleaching or any other complaints from patients such as sensitivity.
Within the limitations of this in vitro study the following conclusions were drawn:
• The application of 10% carbamide peroxide significantly changed the color of the enamel surface and increased its surface roughness.
• The n-CAP surface treatment was significantly effective in reducing the increased surface roughness as the result of bleaching. N-CAP also was able to reduce enamel re-staining after bleaching.
• The application of Flor-Opal and GC Tooth Mousse showed a reduction in surface roughness of bleached enamel. However, no significant difference in surface roughness was found between bleached enamel treated by those two materials and their baseline surface roughness.
• Although ΔE2 of the enamel specimens treated with n-CAP was lower than those treated with Flor-Opal and GC Tooth Mousse, no significant difference in color was found between the n-CAP treated group and the other two groups following staining.
• It is recommended that dentists prescribe these materials, especially n-CAP, to their patients to be used after the bleaching procedure. This is to minimize the undesirable effect of bleaching on enamel such as surface roughness and subsequently staining.
This study was supported and approved by College of Dentistry Research center and Deanship of Scientific Research, King Saud University/Saudi Arabia.
None
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Al-Shamrani A, Awliya W (2019) The Effect of Neutral Sodium Fluoride, Casein Phosphopeptide-Amorphous Calcium Phosphate, and Nano-Carbonate Apatite on Enamel Surface Roughness and Staining after Home Bleaching. Int J Dent Oral Health 5(4): dx.doi. org/10.16966/2378-7090.300
Copyright: © 2019 Al-Shamrani A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access