Abstract
Purpose: A randomized, single-blind, prospective clinical trial was performed to evaluate the effects of plaque control by inter-radicular brushing on class II furcation of lower molars.
Methods: Twenty-two patients presenting with at least one class II furcation with horizontal probing depth (PD) ≥ 4 mm on the buccal aspect of lower molars were recruited. Each patient was randomly assigned to do either inter-radicular brushing with an inter dental brush (test: 12 subjects, 12 sites) or not (control: 10 subjects, 12 sites). After 4 weeks, the clinical parameters of furcated molars were evaluated and microbial analysis of the sub gingival plaque collected from furcation entrances was performed using quantitative real-time polymerase chain reaction.
Results: Two test group patients did not show up at the appointment, and thus, a total of 20 patients completed the study. No statistically significant differences in mean vertical PD, horizontal PD, gingival recession, or plaque index at furcation sites were observed between the control and test groups. However, the mean gingival index of the control and test sites were2.3, and 1.2, respectively, and the mean modified sulcus bleeding index was 2.3 and 0.6, respectively; differences in both sets of parameters were statistically significant between groups. Among total and 6 specific pathogenic bacterial species, the copy numbers of total bacteria, Fusobacterium nucleatum, Parvimonas micra and Tannerella forsythia were significantly lower in test than control sites.
Conclusion: Preventing the accumulation of pathogenic bacteria by inter-radicular brushing can inhibit gingival inflammation and improve the long-term stability of molars with class II furcation defects.
Keywords
Furcation defects; Tooth brushing; Gingivitis
Introduction
Periodontitis is the chronic inflammation of the periodontium caused by plaque biofilm, which destroys tooth-supporting soft and hard tissues [1]. Molar teeth tend to experience more attachment loss than singlerooted teeth and the presence of a furcation lesion reduces the success rate of periodontal therapy [2]. The furcation defect worsens the longterm prognosis of the particular tooth and increases the risk for the tooth loss [3,4]. Therefore, furcation-involved molars have traditionally been assigned a guarded or poor prognosis [5] and are one of the major concerns to dentists who are treating periodontal disease.
The major reason for poor prognosis in the treatment of furcation defect is the incomplete removal of subgingival plaque and calculus in the inter-radicular area [6]. The variable morphology such as cervical enamel projections, bifurcation ridges, convexities, concavities and furcation entrance dimensions can limit the complete debridement of sub-gingival plaque and calculus by the practitioner [6-8]. Moreover, the deep cavernous furcation space makes adequate plaque control difficult for the patients. Thus, accumulation of plaque bacteria by limited access and the resultant inflammatory reaction can provide the ideal environmental niche for accelerating growth of periodontitis-associated microflora. The primary etiological factor for the development of gingival inflammation has been shown to be the accumulation and maturation of bacterial biofilm at the subgingival space [9]. After subgingival instrumentation under the gingival margin, bacteria were re-colonized in the subgingival space. At that time, the non-pathogenic bacteria were preferentially accumulated in the subgingival space immediately after subgingival instrumentation; however, the change in the bacterial composition to pathogenic bacteria was complete after several months [10,11]. Failure to perform oral hygiene adequately at the furcation space can accelerate the accumulation of pathogenic bacteria, which has the potential to initiate gingival inflammation and, in some individuals, to progress to alveolar bone loss. Therefore, daily plaque removal by the patient from the inter-radicular space can be very effective in controlling the gingival inflammation of the furcation area.
Inter dental cleaning with inter dental brushes is the most effective method for inter-dental plaque removal, and thus, it is preferentially recommended to patients with periodontitis [12]. Interdental brushing was shown to remove subgingival plaque that had extended to 2.5 mm below the gingival margin [11]. The size of the Gracey curette is at least 0.75 mm, and thus, it sometimes cannot approach the deep furcation defect [8,13].The size of the core steel of interdental brushes is less than 0.5 mm; therefore, the small size of the interdental brush can effectively reach into the deep inter-radicular space.
Routine tooth brushing alone cannot penetrate and clean the interradicular space. The use of an interdental brush for class III (throughand-through furcation defects) has been recommended [5]; however, the use of an interdental brush for class II defects has not been reported. The purpose of this study is to verify the effect of inter-radicular brushing on buccal furcation class II defects (cul-de-sac) of the mandibular molars.
Materials and Methods
Study subjects
This study was designed as a randomized, single blind, prospective trial. The study protocol was approved by the Institutional Review Board for Human Subjects of the Korea University Anam Hospital (IRB No. ED 12256). For this study, 22 participants were recruited from patients visiting the Department of Periodontology, Korea University Anam Hospital. One of the authors examined each patient to determine whether the patient had class II furcation-involved mandibular molars. Subjects were recruited from January 2013 to December 2014. Patients who had undergone follow-up visits more than 6 months after cause-related therapy were recruited. The patients provided written informed consent. The furcation defects were classified as follows: Furcation class I-less than or equal to 3 mm horizontal depth; Furcation class II-greater than 3 mm horizontal depth but not through-and-through; Furcation class III-through-andthrough involvement [14]. A total of 24 furcated molars were involved. The patients satisfying inclusion criteria were asked for their participation by one of the authors. Any patient with one or more of the following criteria was not admitted to the study: pregnancy, antibiotics or antiinflammatory drugs taken within the last 6 months, drug consumption that may cause gingival enlargement (such as phenytoin or cyclosporine), presence of un-controlled diabetes mellitus, heavy smoking (a pack /day or more), and furcation involvement caused by oral diseases other than periodontitis.
Study designs
After verifying that a patient met the inclusion criteria, the subject was enrolled in the study and given a case number. Examiner I (Lee) performed the randomization of the test/control group using a computergenerated randomization table. Throughout the study, the randomization code was concealed from examiner II (Ji). All participants were instructed to use their original brushing method and to do interdental brushing on interdental spaces. Each patient in the test group received additional instruction as to the correct handling and frequency for use of interdental brushing of the furcation defect by examiner I (Lee). Inter-radicular brushing was performed with a standard interdental brush (TePe, Malmö and Sweden). The interdental brush consisted of a stainless steel metal core with twisted nylon filaments and was available in two sizes for this study consisting of either a 0.45 (orange colored interdental brush) or a 0.5 (red colored interdental brush) mm in metal core diameter. The interdental brush was guided through the inter-radicular spaces of the mandibular class II furcation involvement as far as the interdental brush is limited with light pressure. In situations where the entrance is not exposed to the oral cavity, the patient was instructed on how to insert the interdental brush in the middle of the mandibular teeth. Patients were instructed to try this insertion several times while looking in the mirror. Each participant in the test group was asked to do inter-radicular brushing once per day, before going to bed. When the participants performed inter-radicular brushing, they were required to repeat the insertion and removal of the interdental brush into the inter-radicular space five times. All participants received professional plaque control and the plaque of the furcation area was removed with interdental brushing, EMS ultrasonic scaler, and Gracy curettes.
The effect of inter-radicular brushing was evaluated after 4 weeks by examiner II (Ji), who did not participate in dividing the participants into the control and test groups. The clinical examination for all participants’ dentition included the total number of teeth, plaque index (PI) [15], probing depth (PD), clinical attachment level (CAL), and percentage of bleeding on probing (BOP). The clinical examination of the furcation sites included PI, vertical and horizontal PDs, CAL and gingival index (GI), and modified sulcus bleeding index on probing (Figure. 1). The GI was scored in the buccal furcation according to the following criteria: 0=healthy gingiva; 1=gingiva looks inflamed, but do not bleed when probed; 2=gingiva look inflamed and bleed when probed; and 3=ulceration and spontaneous bleeding [15]. The modified sulcus bleeding index on probing was scored in the furcation according to the following criteria: 0=No bleeding when a periodontal probe is passed along the gingival margin; 1=isolated bleeding spots visible; 2=Blood forms a confluent red line on margin; and 3=Heavy or profuse bleeding [16]. The horizontal PDs of the furcation area were measured with a Nabersprobe (HU FRIEDY,Chicago, IL, USA).
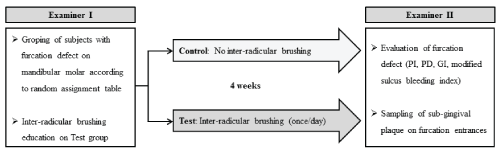
Figure 1: Study design for assessment of the effect of inter-radicular brushing
Examiner I performed the randomization of the test/control group using a computer-generated randomization table and instructed the correct handling and frequency for use of interdental brushing on furcation defects. Each participant of test group was asked to do inter-radicular brushing a time per day, before going to bed. After 4 weeks, the examiner II examined the clinical parameters of participants including furcation sites and sampled the subgingival plaques from furcation space of test and control sites.
PI: Plaque Index; PD: Probing Depth; GI: Gingival Indexs
Microbiological test
Subgingival plaques were obtained from the furcation space of test and control sites by examiner II (Ji). Before plaque collection from the teeth, supra-gingival plaque was removed with a hand curette. Each tooth site was gently dried with compressed air for 10 seconds and isolated from saliva with a cotton roll. Subgingival plaque samples were obtained from the furcation areas using two sterile endodontic paper points (CaulkDentsply, Milford, DE, USA). Paper points were pushed toward the subgingival pocket at the furcation entrance until a slight resistance was felt; they remained in place for 30 seconds. Paper points were placed in a single, labeled tube containing 100 μl of phosphate-buffered saline. The tubes were then transported to the laboratory and stored in a rack into a deep freezer (-80℃). The tubes containing paper points were centrifuged at 12,000 rpm at 4°C for 5 min, and the precipitated pellets were sent to CytoGen Co. (Cytoperio, Korea, www.4hwell.com) for quantitative analysis of the subgingival bacteria. Quantitative real-time PCR (qRTPCR) was performed for the quantitative analysis of total bacteria, 3 orange complex bacteria (Fusobacterium nucleatum, Parvimonas micra, and Campylobacter rectus), and three red complex bacteria (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola).
Statistical analysis
Comparisons between control and inter-radicular brushing groups were analyzed using the Mann-Whitney U-test. Analyses were performed using SPSS (version 19, SPSS, Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Study subjects
Two patients assigned into test groups did not show up for their appointments for reasons not related to the study; thus, a total 22 furcation defects from 20 patients were analyzed in this study. Ten sites in 10 subjects of the test group and 12 sites in 10 subjects of the control group were analyzed. The data indicated that the control and test groups were similar in terms of age, total number of teeth, PI, PD, CAL, and percentage of BOP (Table 1). The mean plaque indices of the control and the test subjects were0.39 and 0.26, respectively, and the mean percentages of BOP were38.78% and 35.76%, respectively (Table 1).
| |
Control(n=10 subjects) |
Test(n=10 subjects) |
P-value |
| Gender (Male/Female) |
7/3 |
6/4 |
|
| Mean Age (years) |
54.6 ± 2.02 |
51.4 ± 2.82 |
0.228 |
| Mean total tooth count |
26.1 ± 1.47 |
27.7 ± 0.15 |
0.254 |
| Mean PI |
0.39 ± 0.09 |
0.26 ± 0.04 |
0.18 |
| Mean PD (mm) |
2.77 ± 0.09 |
2.86 ± 0.12 |
0.456 |
| Mean CAL (mm) |
4.09 ± 0.34 |
3.81 ± 0.10 |
0.283 |
| Mean percentage of BOP (%) |
38.78 ± 2.76 |
35.76 ± 3.67 |
0.821 |
Table 1: The clinical characteristics of subjects
PI: Plaque Index; PD: Probing Depth; CAL: Clinical Attachment Level; BOP: Bleeding on Probing
The evaluation of furcation defects
This study included 9 first and 3 second mandibular molars in the control group and 8 first and 2 second mandibular molars in the test group. The mean vertical PDs of the control and the test sites were 4.1 and 4.2 mm, respectively, and the mean horizontal PDs of the control and the test sites were5.4 mm and 5.4 mm, respectively. The mean CALs of the control and the test sites were 5.8 mm and 6.1 mm, respectively. The mean plaque indices of the control and the test sites were 0.4 and 0. No statistically significant differences with regard to mean vertical PD, horizontal PD, gingival recession, CAL, or PIexisted between control and test groups. However, the mean GI of control sites was 2.3, whereas that of test sites was 1.2; the difference between control and test sites was statistically significant (p=0.007). Moreover, the mean modified sulcus bleeding index of control sites was 2.3, whereas that of test sites was 0.6; this difference was also statistically significant (p<0.0001 [Table 2]). The sample size was calculated using mean and standard deviation of modified sulcus bleeding index, the primary outcome of this study. Group sample sizes of test 7 and control 7 achieved 96.671% power to reject the null hypothesis of equal means when the difference in population means (μ1- μ2, 0.6-2.2) was -1.6 with standard deviations of 0.70 for test and 0.72 for control group, and with a significance level (alpha) of 0.050 using a twosided two-sample unequal-variance t-test. When the dropout rate was considered to be 30%, the sample sizes of each group were 10. Therefore, the comparison between 10 sites in test group and 12 sites in the control group was sufficient to prove the effect of inter-radicular brushing. The effect size was also analyzed using mean and standard deviation of modified sulcus bleeding index. The Cohen’s d was 2.2 and it showed that the inter-radicular brushing effect can be large [17].
| |
Control (n=12 sites) |
Test (n=10 sites) |
P-value |
| Mean vertical PD |
4.33 ± 1.67 |
4.2 ± 1.55 |
0.923 |
| Mean horizontal PD |
5.33 ± 1.15 |
5.4 ± 0.7 |
0.974 |
| Mean gingival recession |
1.67 ± 0.89 |
1.9 ± 0.99 |
0.539 |
| Mean CAL |
6.0 ± 1.76 |
6.1 ± 2.13 |
0.923 |
| Mean PI |
0.33 ± 0.49 |
0 |
0.203 |
| Mean GI [15] |
2.33 ± 0.49 |
1.2 ± 0.92 |
0.007a) |
| Mean modified sulcus bleeding index [16] |
2.17 ± 0.72 |
0.6 ± 0.70 |
0.000a) |
Table 2: The clinical evaluation of furcation sites
PD: Probing Depth; CAL: Clinical Attachment Level; PI: Plaque Index; GI: Gingival Index
a)Statistically significant differences between groups (Mann-Whitney U-test, p<0.01).
The microbial analysis of furcation defects
The copy number of total bacteria, 3 orange complex bacteria, and 3 red complex bacteria was analyzed. All examined bacterial copy numbers tended to be lower in the inter-radicular brushing test group than in control group. Among them, the copy number of total bacteria, F. nucleatum, P. micra, and T. forsythia were significantly lower in the test group than in the control group (Figure 2). Moreover, 5 bacterial species except F. nucleatum, were less frequently detected in the test sites than in the control sites. P. micra and C. rectus were detected in 9 and 6 sites, respectively, among a total of 10 test sites, while both bacteria were detected in all control sites. Moreover, P. gingivalis, T. denticola, and T. forsythia were only detected in 5, 5, and 6 test sites, respectively, whereas they were detected in 8, 9, and 11 control sites, respectively. F. nucleatum was detected in all test and control sites.
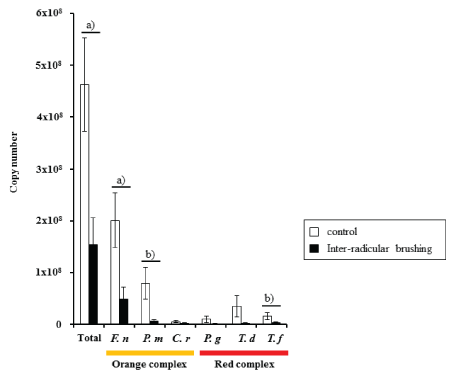
Figure 2: The microbial analysis of subgingival plaque from furcation defects
Subgingival plaques were obtained from the furcation space of test and control sites andthe copy numbers of total bacteria, 3 orange and 3 red complex bacteria were analyzedusing quantitative real-time polymerase chain reaction. F. n; F. nucleatum, P. m; P. micra, C. r; C. rectus, P. g; P. gingivalis, T. f; T. forsythia, T. d; T. denticola.
a)Statistically significant differences between groups (Mann-Whitney U-test, p<0.01).
b)Statistically significant differences between groups (Mann-Whitney U-test, p<0.05).
Discussion
The existence of deep, tortuous pocket and concavity-associated furcation lesions favours the colonization of pathogenic bacterial species. These lesions are associated with gingival inflammation and bone loss. Inter-radicular brushing using an inter-dental brush of a class II furcation defect appears to reduce subgingival bacterial frequency and count and also reduce gingival inflammation as determined by the GI and modified sulcus bleeding index. Therefore, inter-radicular brushing may reduce the possibility of bone loss caused by gingival inflammation.
The anatomic limitation of furcation areas is reported to considerably increase the risk for tooth loss [6-8,13]. Studies reporting that the efficacy of calculus removal at furcation areas showed that the portion of the calculus still remained, even though the furcation area received flap surgery [6,18]. However, the rough surface of the calculus does not in itself induce inflammation, but the calculus’s deleterious effect relates to its ability to provide an ideal surface for microbial colonization [19]. It has also been demonstrated that epithelial adherence to subgingival calculi can occur following its disinfection with chlorhexidine [20]. Interradicular brushing can mechanically remove the plaque over a calculus even if the calculus still remains in the furcation area. Moreover, interradicular brushing can overcome the limitations caused by the anatomic characteristics of furcation defects. One of major causes of difficulty during treatment is a narrow furcation entrance. In a study analyzing 114 maxillary and 103 mandibular first molars, half of all furcation entrance dimensions of molars were less than the blade width (0.75 mm) of a Gracey curette [8]. Meanwhile, the central wire’s diameter of the smallest interdental brush (Tepe) is 0.4 mm, which is less than the blade width of a Gracey curette. Therefore, inter-radicular brushing can be effectively inserted into the narrow entrance of furcation defects. In addition, the concavity of the furcal aspect was present in 100% and 99% of mandibular mesial and distal roots, respectively [8]. Once the plaque front has reached the furcation area, these concave configurations render plaque control quite difficult. However, inter-radicular brushing can remove the plaque on the concave surfaces due to the structural characteristics of the interdental brush itself. Moreover, cervical enamel projections extending from the cemento-enamel junction into the furcation cannot be a problem to do inter-radicular brushing. Therefore, inter-radicular brushing can help plaque removal in the furcation space by overcoming several anatomic limitations of the furcation area.
It is well known that class II furcation defects on mandibular molars can be a site to try for a regenerative approach [21]. Various approaches for regeneration at furcation defects have been reported [21]. A recent meta-analysis showed that guided tissue regeneration (GTR) resulted in significantly greater horizontal-CAL gains and horizontal bone fills than flap surgery. Regarding horizontal-CAL gain in mandibular molars, the weighted mean difference between GTR and flap surgery was 1.15 mm (in favor of GTR) and between GTR + bone graft and flap surgery was 1.76 mm (in favor of GTR + bone graft). Regarding horizontal bone fill in mandibular molars, the weighted mean difference between GTR and flap surgery was 1.55 mm and between GTR + bone graft and flap surgery was 1.34 mm (in favor of GTR + bone graft) [22]. These results indicate that mandibular class II furcation may benefit from GTR treatment. However, the complete elimination of a furcation defect rarely occurs, and partial furcation defects still remain even though GTR has successfully been achieved [23]. Moreover, reports evaluating the efficacy of GTR showed that the GTR technique is technique-sensitive and less predictable if the treatment objective is the complete resolution of the furcation involvement [23]. Tunnel preparation is another approach used to treat deep class II defects in mandibular molars [5]. Although tunnel preparation is considered to be able to secure inter-radicular space for plaque control, it often requires resection of the alveolar bone around the furcation area. Moreover, tunnel preparation should be used with caution because the denuded root surfaces within artificially prepared tunnels have the possibilities of root sensitivity and caries [24]. By contrast, inter-radicular brushing is a conservative, non-surgical, non-techniquesensitive approach that dentists can easily suggest to their patients.
In our study, the copy numbers of total bacteria, F. nucleatum, P. micra, and T. forsythia were significantly lower in test sites than in control sites, and P. micra, C. rectus, and red complex species were less frequently detected on subgingival plaque samples from the inter-radicular brushing group. It was previously demonstrated that the meticulous and prolonged supra-gingival plaque removal reduced the total number of microorganisms at sites with supra- and intra-bony pockets as well as at furcation sites [25].Inter-radicular brushing can additionally affect microbial biofilm composition inside the furcation defect.
However, this study did not consider that each individual could have various counts and proportions of bacterial species. If this study was designed to compare before and after inter-radicular brushing considering individual variability effects on proportions and counts of bacterial species, microbial analysis would provide a clear effect of inter-radicular brushing on the microbial biofilm composition. Moreover, the change in CAL and bony stability in the class II furcation area should be evaluated through long-term performance of inter-radicular brushing.
Longitudinal studies showed that molars with furcation involvement have an acceptable long-term survival rate although the prognosis in furcated molars is not as satisfactory as those obtained for singlerooted teeth or non-furcated molars [3,26,27]. Prevention of pathogenic bacterial accumulation by inter-radicular brushing can inhibit gingival inflammation and improve the long-term stability of molars having class II furcation defects.
Conflict of Interest
No conflict of interest exists.
Acknowledgement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education, Science and Technology (NRF- 2016R1D1A1B03931792).



