Figure 1: Flow chart


Paulo Sandoval1* Betty Bizcar2 Pablo Navarro3 Michael Knosel4
1Professor of Orthodontics, Program Chair, Universidad de La Frontera, Temuco, Chile*Corresponding author: Paulo Sandoval, Professor of Orthodontics, Program Chair, Universidad de La Frontera, Temuco, Chile, E-mail: paulo.sandoval@ufrontera.cl
Purpose: To test the null-hypothesis of no significant difference in velocity of space closure of extraction in sectors undergoing laser bio stimulation and those that were not exposed.
Methods: Using an incomplete block split-mouth design, the quadrants of twenty patients requiring extraction of first premolars were randomly assigned to an intervention (laser exposure) and a control (non-exposure to laser) group. Space closure and tooth movement were achieved reciprocally by using a lace-back force system between anterior and posterior teeth, assessed with digital caliper. The experimental side was subject to infrared radiation from a semiconductor (aluminium gallium arsenide) diode laser with a wavelength of 940 nm on days 0,7, and 14 in the first month, and thereafter on every 15th day until complete canine retraction was achieved on the experimental side.
Results: An average increase of 30% in the rate of tooth movement was observed with the low-intensity laser therapy compared with the control sides. Gap closing speed between intervention and control sides were significantly different following day 14.
Conclusion: The null-hypothesis was rejected. Low-intensity laser therapy with the settings used here is considered as a valid complementary option to accelerate orthodontic space closure.
Laser; Tooth movement; Low-level laser therapy
A major concern to orthodontic patients is treatment time. Generally, the time required for fixed appliance treatment ranges from 20 to 30 months. Reducing treatment time requires increasing the rate of orthodontic tooth movement [1].
Many studies have examined different methods that can increase the rate of orthodontic tooth movement, including local injections of: prostaglandins [2,3] ,1, 25(OH) 2 D3 [4] (the active form of vitamin D3 ) but were recently rejected [5],osteocalcin [6],and relaxin [7] around the alveolar socket. Although these substances stimulate the rate of tooth movement, they also can lead to the undesirable side effects of local pain and discomfort during injections.Later resonance vibration [8] has been tried on animals, but these methods require an apparatus that is not routinely used in dental practice. Recently Acceledent(R) was reported effective in increasing the rate of tooth movement when applied as an adjunct to orthodontic treatment [9]. But another prospective randomized clinical trial found no evidence that supplemental vibrational force can significantly increase the rate of initial tooth movement and the device requires strict compliance by the patient [10].
Low-intensity laser therapy has an energy output that is low enough so as not to cause the temperature of treated tissues to rise above 36.5°C or normal body temperature [11]. Initially, low-intensity laser therapy was applied only in medical sciences such as orthopedics, surgery, and medicine. It is used to accelerate the callus formation at fracture sites to facilitate wound healing [12]. Recently, LLLT suggested its use for distraction osteogenesis [13]. In the last decade, many histologic studies have attempted to determine the effect of low-intensity laser therapy on the histochemical pathways directly associated with orthodontic tooth movement [14-18]. Increased osteoblastic and osteoclastic activity after low-level laser therapy was observed in vivo and in vitro, but much of this research wascarried out in short-term studiesonanimals [19]. Few studies in literature have examined the clinical effects of low-level laser therapy on the rate of orthodontic tooth movement [20-23,19] and results were found as controversial.
The aim of this study was to evaluate the effect of a new dose of lowlevel laser therapy on the speed of tooth movement during orthodontic treatment. We tested the null hypothesis of no significant difference in velocity of space closure of extraction in sectors undergoing laser biostimulation and those that were not exposed.
A sample of 20 healthy orthodontic patients (8 males, 12 females; aged 18-25) from a private practice in Temuco Chile in was recruited adopting the inclusion criteria of
Exclusion criteria were as follows:
Sample size was determined by power analysis based on the results of a previous study that showed that the rate of tooth movement was twice that of the control side [21]. With an allowed error of 5% by using a split-mouth design, a sample size of 20 was sufficient for 80% power and achievement of significance. Informed consent to laser irradiation was obtained from each patient.
Orthodontic diagnostic records were collected and analyzed for all subjects, including clinical screening of the vitality of the periodontium by pocket depth probing prior to and following space closure. The treatment plans for the patients included extraction of the maxillary first premolars to meet the space requirements for the retraction of anterior teeth.
Incomplete block split-mouth design was used to prevent inter individual biologic variation [24]. In the 20 patients, maxillary first premolars were extracted. In each patient, the extracted right and left quadrants were randomly divided into two groups. Patients were blinded about the experimental and control sides. Group 1 was the experimental side and received laser therapy. Group 2 was the control side quadrant and did not receive low-intensity laser therapy
Pre-adjusted edgewise MBT brackets (0.022-in; Ortho Organizers, Carlsbad, Calif, USA) were bonded using light-cured Transbond XT (3M Unitek, Monrovia, CA, USA). Archwire sequence was 0.014-in heatactivated nickel-titanium for alignment and levelling, followed by0.018- in, 0.017 × 0.025-in and 0.019 × 0.025-in nickel-titanium. A 0.019 × 0.025-in stainless steel (Permachrome Wire; 3M Unitek, Monrovia, CA, USA) was used during space closure: Twenty-one days following steel wire placement, retractionin masse with reciprocal anchorage was started with alace-back (3M Unitek, Monrovia, CA, USA). Incisors were consolidated by using 0.009-in steel ligature wires. The second premolar, the first and second molar were also consolidated to achieve an anchorage unit. A force of 150g was used for group retraction on both the control and experimental sides, in order to maintain the clinical protocol proposed by McLaughlin, Bennet and Trevisi (MBT system) [25]. The exerted force value was confirmed with an orthodontic dynamometer by two dentists. Patients were asked to report immediately if the lace-back dislodged or broke. In none of the patients the lace-back detached.
Low-intensity laser therapy was started on the randomly selected experimental side on the same day as the lace-back.
The laser type used was a AlGaInAs semiconductor diode (EPIC X, Biolase Technology, Inc., Irvine, CA) emitting infrared radiation with a wavelength of 940 ± 10 nm operated according to the manufacturer’s recommendations. All safety precautions for the patient and the operator were followed. The aforementioned medical equipment has a wide range of settings according to the required treatment.
The hand piece had a cylindrical quartz tip with a surface area of 0.4 mm2 where the laser beam was emitted from. The black color-coded needle (used for therapeutic purposes) was attached to the hand piece for lowintensity laser therapy. Routine method of sterilization and disinfection was followed. In particular, the hand piece body and the optic tips were sterilized by cold sterilization.
Protection glasses were worn by both the operator and the patient. These glasses, provided by the manufacturer, were in accordance with the European norm EN 207 and had an optical density of ≥ 5 at the wavelength of emission from the diode.
Low-intensity laser therapy was started for bio-stimulation on the day of lace-back placement. Irradiation was performed on the canine and second premolar holding the laser tip without direct tissue contact. A total of 12 irradiation areas were covered: three on the buccal side and three on the palatal side of each tooth. The laser regimen was applied on day 0, 7, and 14 in the first month (T0, T1, T2). Thereafter, irradiations were carried out on every 15th day until complete canine retraction on the experimental side was achieved (T3, T4, T5, T6).
To cover all periodontal fibres and bone around canines, distribution and order were as follows: on the buccal side, there were;
For the second premolars, distribution and order were as follows: on the buccal side, there was
On the palatal side, irradiations were performed similarly. The tip was not in contact with the tissue during application. This procedure was followed for all subsequent appointments. The total energy density (dose) at each application was 6 J (2 × 30 s × 100 mW).
To avoid intra-operator variation, all irradiations were performed by the same operator. To control patient behaviour and to maintain blindness, the hand piece was also held on the control side just to project the red guiding light with no laser emission. After 6 months, the laser side (experimental) and the control side were examined by using panoramic radiographs, which showed no undesirable changes in the adjacent periodontal ligaments and alveolar bones.
At least sixassessments were made for each patient. The tips of the mesial cusp of the first molar and the canine were used asintra-oral reference points. The assessor was blinded to control or experimental sides. That is, one person measured the two quadrants without knowing which was irradiated with laser. Distance between the second premolar and the canine was measured for each patient with a digital calliper (Aerospace, Shanghai, China) accurate to ± 0.02 mm directly in mouth between contact point and tip cuspid. The distances were recorded at T0 (after completion of alignment and levelling: day 1 of canine retraction), T1 (at the end of 1 week of canine retraction) to T6 (on completion of group retraction on the experimental side).
Master sheets were prepared to facilitate analysis of the data. Data were tabulated and analysed by statistical software (version 20.0; SPSS, Chicago, IL, USA). The descriptive statistics of mean differences, standard deviations, and standard errors were calculated for all variables (Table 1).
| Experimental | Control | |
| T0 | 5.87 ± 0.70 | 5.89 ± 0.64 |
| T1 | 5.45 ± 0.65 | 5.67 ± 0.62 |
| T2 | 4.81 ± 0.68 | 5.31 ± 0.62 |
| T3 | 3.58 ± 0.64 | 4.34 ± 0.59 |
| T4 | 2.34 ± 0.65 | 3.39 ± 0.60 |
| T5 | 1.12 ± 0.61 | 2.47 ± 0.59 |
| T6 | 0.59 ± 0.37 | 1.36 ± 0.54 |
| P value | <0.0001 | <0.0001 |
| Inference | S | S |
Table 1: Distances (mm) between the distal side of canines and the mesial
side of the first premolars in maxillary arch
ANOVA: S: Statisticly significant
The paired t test for related samples Wilcox test was used to compare the variables within the groups, previous Shapiro-Wilk test for normality. One-way analysis of variance (Repeated measures ANOVA; F statistics) was used to comparee the distances at T0, T1 to T5 in both groups. Multiple comparisons were made with the Bonferroni test. After analysis, data were sorted into various tables based on the objectives of the study. Results are expressed as levels of significance. Significance was determined at 0.05 level of confidence.
Prior to space closure, the sides did not differ significantly in terms of distances between the canines and premolars, so the distribution of extraction gaps was considered to be homogeneous based on the ShapiroWilk test that was used to test the normality of the data.
Descriptive statistics are presented in table 1 and the difference between T0 and T5 was the amount of tooth movement over the period of 42 days in table 2.
| Variable | Experimental group | Control group | t value | P value | Inference |
| T0-T3 | 2.29 ± 0.46 | 1.55 ± 0.45 | 2.1458 | <0.0001 | S |
| T0-T5 | 4.75 ± 0.46 | 3.42 ± 0.45 | 4.9156 | <0.0001 | S |
Table 2: Amounts (mm) of canine retraction in the control and experimental
sides
S: significant statisticly; T0-T3, amount of canine retraction in 1 months; T0-
T6, amount of space closure in 2.5 months. *Paired t test applied
Rate of orthodontic tooth movement was calculated as the amount of tooth movement divided by the time period. The rate of orthodontic tooth movement at the end of 1 month (M1) was recorded as T0 – T3 divided by 28 days.
Rate of orthodontic tooth movement on medium of canine retraction (M2) on the experimental side was recorded as T3 – T5 divided by 28 days. The M1 and M2 readings were calculated for both the experimental and control sides and compared (Table 3).
| M1 (mm/d) | M2 (mm/d) | |||||
| Control | Experimental | Result | Control | Experimental | Result | |
| Maxillary | 0.05 ± 0.55 | 0.09 ± 0.15 | HS | 0.07 ± 0.21 | 0.09 ± 0.22 | S |
Table 3: Comparison of the rates of space closure
S: Significant statisticly; M1: Rate at end of 1 months; M2: Rate at medium of space closure
To calculate errors in measurements, they were repeated by another operator. Mmeasurement error was calculated according to Dahlberg’s formula [26]. This difference was within 0.05 mm; meaning that it was insignificant.
The values of M1 were 0.97 mm per month on the control side and 1.23 mm per month on the experimental side. The values of M2 were 0.92 mm per month on the control side and 1.22 mm per month on the experimental side (Table 3). There was a highly significant positive difference in the rates of tooth movement on the experimental side compared with the control side (Figure 1). The average increase in the rates of tooth movement at 1 month was 32.3% in the maxillary arch and at second month it was 28.0% (Figure 2). The average increase in the rate of tooth movement after anterior retraction was 30% in the maxillary arch. Clinical assessment of the trial or control teeth did not reveal deterioration in terms of periodontal conditions or vitality following space closure (Figure 3).

Figure 1: Flow chart
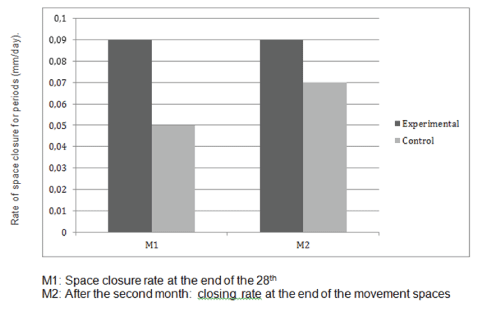
Figure 2: Rates of tooth movement in the control and experimental groups
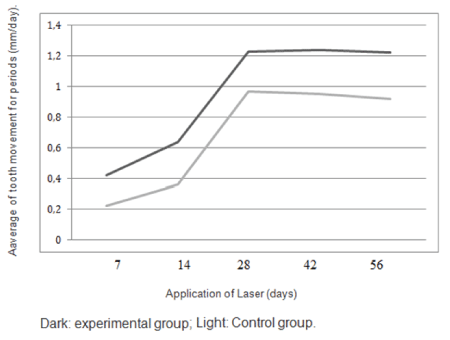
Figure 3: Movement speed for space closure in the intervals in the control and experimental groups
In this study, we used the semiconductor with a wavelength of 940 nm, a pulsed wave mode, an output power of 1mW, and an exposure time of 10 seconds. Results of Bradley et al. [27] had indicated significant biostimulatory effects on bone metabolism around this dosage, whereas higher dosages had bio-inhibitory effects, and lower dosage showed non significant results. A 15-day regimen was used, coinciding with normal recall visits for hygienist control in clinical service. Kavaliauskiene et al. [28] proved that after an orthodontic procedure, pain and soreness occur after 24 to 48 hours. Thus, the first follow-up score after low-intensity laser therapy was recorded on day 7.
A direct comparison between this study and previous studies was limited by a number of factors, such as different laser parameters and various animal models. Some researchers omitted descriptions of important aspects related to the study designs. The force to move teeth also differed across studies, as did the methods used for measuring movement.
The rate of tooth movement after 3 months on the experimental side showed a 1.3fold increase, which equals a rate of 30% more compared with the control side. Findings reported by Kawasaki and Shimizu [29] and Yoshida et al. [30] were greater: 54%. They reported a 1.3-fold increase in movement in their experimental laser group over periods of 12 and 21 days in rats. Fujita et al. [15] also demonstrated a 1.5-fold increase in their irradiated group over only 7 days. However, their study was on rat molars, and orthodontic force was not specified.
Kim et al. [31] reported a higher rate of tooth movement than that in our study over a period of 2 months in dogs. They found a 2.08-fold increase in tooth movement for their experimental low-intensity laser therapy sample, compared with a 1.3-fold increase over 3 months in our study. They used a pulsed mode similar to that used in our study. Yoshida et al. [30] stated that laser units show more bio-stimulatory response when functioning in pulsed mode, but Bradley et al. [27] used the continuous mode effectively. The continuous mode also has peaks and valleys because the laser unit cannot emit continuously in real time.
Cruz et al. [20] were the first to carry out a human study on the effect of low-intensity laser therapy on orthodontic tooth movement. They showed that irradiated canines were retracted at a rate 34% greater than the control canines over 60 days. Similar to our study, the rate of retraction was 30% greater on the experimental side but over a period of 3 months.
Goulart et al. [32] carried out a split-mouth study on dogs subdivided into three groups, one control and two experimental. In one experimental group, they used an energy density of 5.25 J per square centimetre; in the second experimental group, they used 35 J per square centimetre. The first experimental group recorded a 50% increase, but the second experimental group reported a 90% decrease during the first 21 days. They concluded that low-intensity laser therapy with an energy density of 5 J per square centimetre and a total dose of 1.89 J had a stimulatory effect when compared with the controls.The energy density of 35 J per square centimetre and a total dose of 12.6 J had a bio-inhibitory effect when compared with the dog controls. In our study, an energy density of 5 J per square centimetre and a total dose of 6 J were used.
Findings of Limpanichkul et al. [23] differed from ours. They showed no difference between the experimental low-intensity laser therapy subjects and the controls in a split-mouth study on human beings over 4 months. The reason could be the higher energy density of 25 J per square centimetre that they used. The average time required for canine retraction on the experimental side was 4.5 months. Thus, the rate on the experimental side was 1.5 times, or 30%, more compared with the control side. However, Youssef et al. [19] reported a rate of canine retraction almost twice as fast as that of the control canines over a 6-month period on human subjects using a split-mouth design. The reason could be the increased irradiation frequency of four times per month until the end of canine retraction compared with 3 times per month in the first month and two times per month in subsequent months in our study.
In this study, in the first 2 months, a 32% increase in rate of tooth movement was observed, whereas the average increase during the entire period of canine retraction was only 28%. This indicates a decrease in the rate of tooth movement in later time periods; this could be explained as analogous to the normal lag phase in orthodontic tooth movement.
Doshi-Mehta and Bhad-Patil [21] compared the rate of orthodontic tooth movement in the maxillary and mandibular arches. In their study, the average increases in the rates of tooth movement at 3 months were 54% in the maxillary arch and 58% in the mandibular arch. Average increases in the rates of tooth movement after canine retraction were 29% in the maxillary arch and 31% in the mandibular arch. The above differences were not statistically significant but clinically thought-provoking. The smaller increase in the maxillary arch might be due to the periodontal ligament of the canines being farther from the site of irradiation on the palatal side. Esnouf et al. [33] showed a significant reduction in intensity in the first millimetre of penetration: ie, up to 66%. The clinical implication will be that more energy density should be used on the palatal surfaces of the maxillary teeth.
Tooth movement speed is subject to wide variation. Therefore, the generalization of individual studies and comparisons of the results of the study are limited: For example, subjects brush their teeth differently and although we give a reminder at every session, so there could have been some inconsistencies. Also, other variations in dietary habits or other factors may constitute potential sources of deviations in speed levels. Variation in patient age ranges was not considered separately during the analysis as we focused on young patients aged 18-25. Although a sample of 20 subjects is statistically enough, the quality of dental extractions can be subject to variation, and this factor should be considered as a potential source of bias in speed space closure. Despite great care was dedicated to ensure that the forces were 150g, saliva may have affected the discharge capacity of the force among individuals, as reported by different authors [34,35]. In addition, the use of lace-backs, which is a common practice among orthodontists using MBT technique, produces an inconstant force, which is a weakness of this design. There is a potential systemic effect of laser therapy in different areas of the mouth, but there is no evidence that this actually happens. Although statistical analysis can provide significant differences between laser intervention and control teeth, it is up to clinicians to decide themselves about clinical relevance in their patients.
Low-level laser therapy increases the rate of orthodontic tooth movement in a physiologic manner.
Clinical assessment of the trial or control teeth did not reveal deterioration in terms of periodontal conditions or vitality following space closure.
Thus, low-level laser therapy can be recommended as a safe routine treatment in order to accelerate orthodontic space closure.
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.” In Research protocol Folio number N° 042/2015.- from Universidad La Frontera, Temuco, Chile. “Informed consent was obtained from all individual participants included in the study.”
None
Download Provisional PDF Here
Article Type: Research Article
Citation: Sandoval P, Bizcar B, Navarro P, Knösel M (2017) Efficacy of Diode Laser Therapy in Acceleration of Orthodontic Space Closure: A SplitMouth Randomized Clinical Trial. Int J Dent Oral Health 3(2): doi http://dx.doi.org/10.16966/2378- 7090.229
Copyright: © 2017 Sandoval P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access