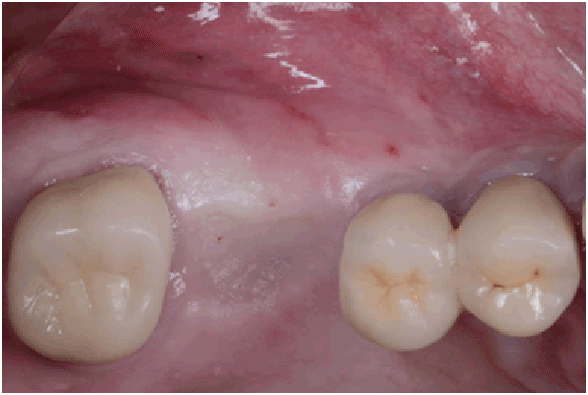
Figure 1a: Pre op: Occlusal view

Dagba AS1,2 Mourlaas J2 Ochoa Durand D2,3 Suzuki T2* Cho SC2 Froum SJ2
1Private Practice, Paris, France*Corresponding author: Suzuki Takanori, Clinical Assistant Professor, Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, USA, E-mail: taka@nyu.edu
In the posterior maxilla insufficient alveolar bone height and proximity of the sinus floor are often limitations for implant placement. Maxillary sinus augmentation has demonstrated a high success rate in the literature and is currently routinely used to enable implant placement in an atrophic posterior maxilla. However, the technique has a potential to develop complications, which could affect and jeopardize the final outcomes. Perforation of the Schneiderian membrane is the most frequently reported complication with an incidence ranging from 10 to 44%. Several protocols have been established in order to repair sinus membrane perforations. Nevertheless these solutions might increase the length of the surgery and are technique sensitive. The purpose of this case series is to present a simple novel technique to treat large sinus perforations of the sinus membrane (>10 mm) during lateral approach sinus floor elevation with a collagen sponge, associate with a re-entry surgery 3-6 weeks following the perforation.
Sinus; Maxillary sinus augmentation; Perforation; Sinus complication; Collagen; Sinus membrane repair
In the posterior maxilla insufficient alveolar bone height and proximity of the sinus floor are often limitations for implant placement [1]. Sinus augmentation by the lateral wall approach is a technique often utilized to increase bone height and enable the placement of conventional length implants. Maxillary sinus augmentation has demonstrated a high success rate in the literature and is currently routinely used to enable implant placement in an atrophic posterior maxilla [2-4]. However, the technique has a potential to develop complications, which could affect and jeopardize the final outcomes. Perforation of the Schneiderian membrane is the most frequently reported complication with an incidence ranging from 10 to 44% [5-8]. Maxillary sinus membrane perforation can lead to loss of graft material, infection, edema, sinus pathology, wound dehiscence and procedure failure.
Several protocols have been established in order to repair sinus membrane perforations. One approach is to re-establish the container function of the membrane with a material that can be either a biomaterial such as a barrier membrane or a biologic material like a subepithelial connective tissue graft. Another approach is to repair the membrane itself. That can be achieved mechanically by the use of sutures or chemically utilizing fibrin glue. Nevertheless these solutions might increase the length of the surgery and are technique sensitive.
Collagen is the most abundant structural protein in mammal’s connective tissue. It is a component of cartilage, cornea, blood vessels, muscles, tendon, bone, and teeth. The three-dimensional structure of collagen allows it to be a fibrous structural protein that provides good mechanical properties. Collagen is a biomaterial utilized in dentistry as a wound dressing and barrier membrane [9]. Because of its excellent cell compatibility and its low antigenicity, it can be used as a scaffold for tissue engineering and enhance tissue regeneration. Moreover, collagen has demonstrated an excellent haemostatic ability and gets resorbed within a short-term period [10]. For these reasons a collagen sponge might be a reliable biomaterial to enhance healing of a perforated Schneiderian membrane. Indeed, the authors achieved successful lateral approach sinus floor elevation (LASFE) within a re-entry surgery 3 to 6 weeks after a previous LASFE. During that initial surgery, following a perforation, a collagen sponge was placed between the wounded membrane and the medial side of the lateral bone wall. To our knowledge no report of such approach has been published in the dental literature.
The purpose of this case series is to present a novel technique to treat large sinus perforations of the sinus membrane (>10 mm) [11] during LASFE with a collagen sponge, associate with a re-entry surgery 3-6 weeks following the perforation.
Clinical data in this study are obtained from the Implant Database (ID) at New York University College of Dentistry (NYUCD). This dataset was extracted as de-identified information from the routine treatment of patients at the Ashman Department of Periodontology and Implant Dentistry at NYUCD. The study was granted exempt status by NYUCD Review Committee based on the fact that the research involved the Department of Periodontology and Implant Dentistry de-identified ID. The ID was certified by the office of Quality Assurance at NYUCD and met all institutional Review Board (IRB) and Health Insurance Portability and Accountability Act requirements.
The dataset for this case series consisted of a pool of patients who had received sinus augmentation using the lateral window approach technique, and for whom large Schneiderian membrane perforation occurred (>10 mm) and were reported (Figures 1a-1e). In each of these patients, <5 mm of crestal bone had to be present below the sinus floor for the patient to be considered for inclusion in this study. A total of 8 patients (10 sinuses, 1 female, 7 males) were selected, age ranged from 35 to 75years (mean: 53.5 years).

Figure 1a: Pre op: Occlusal view
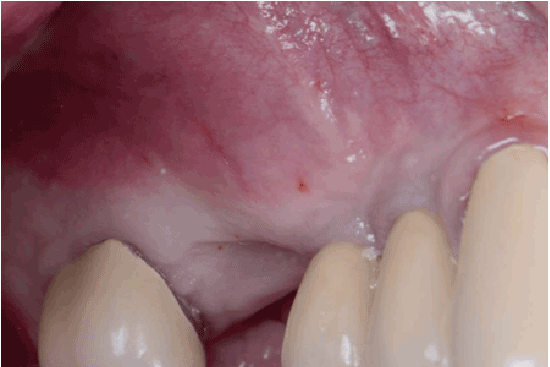
Figure 1b: Pre op: Lateral view
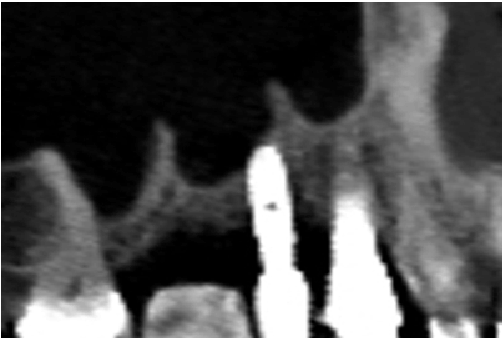
Figure 1c: Pre op: CBCT slide of the surgical site
Exclusion criteria for this study included: 1) patients who could not undergo standard oral surgery procedures for any reasons; 2) patients who smoked >10 cigarettes per day; and 3) females who were pregnant or nursing a child (Table 1).
All patients selected for inclusion had received an antibiotic regimen of 2.0 g amoxicillin given 1 hour before surgery and 500 mg three times daily thereafter for 7 to 10 days. The exception included patients allergic to penicillin, who received 600 mg clindamycin given 1hour before surgery and 150 mg twice daily thereafter for 7 to 10 days. All patients were instructed to rinse with 0.12% chlorhexidinegluconate twice daily for 2 weeks after the surgery.
All perforations (>10 mm) were repaired during the surgery with absorbable collagen membranes (CollaTape®, Integra LifeSciences Corp., Plainsboro, NJ) positioned between the lateral wall of the sinus and the perforated Scheiderian membrane after extension of the bony window (Figure 1f). Primary closure of the flap was achieved with absorbable sutures (4-0 Chromic Gut, Johnson & Johnson, New Brunswick, NJ) (Figures 1g and 1h). Then a sinus re-entry procedure was performed after 3 to 6 weeks of healing time to achieve the sinus augmentation procedure. A full-thickness flap was elevated exposing the initial bony window where a dissection of the soft tissues was required (Figure 2a) to be able to perform the elevation of the healed Schneiderianmembrane (Figure 2b). Since no graft material was used to fill the sinuses during the previous surgeries, the sinuses were then grafted with Anorganic Bovine Bone Matrix (ABBM) (Bio-Oss® , GeistlichPharma, Princeton, NJ) (Figures 2c and 2d) and an absorbable membrane (Bio-Guide® , GeistlichPharma, Princeton, NJ) was placed above the window.
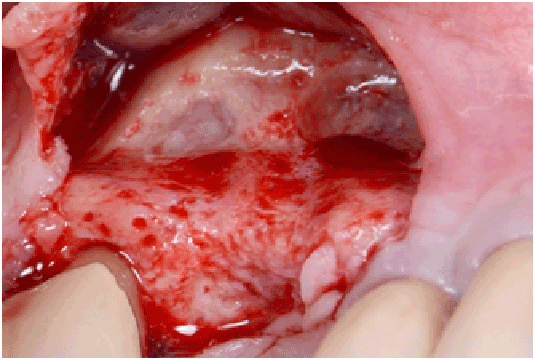
Figure 1d: Flap elevation in order to expose the sinus surgical site
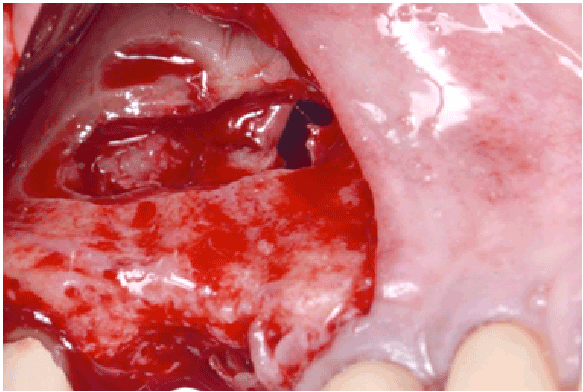
Figure 1e: Perforation of the Schneiderian membrane >10 mm
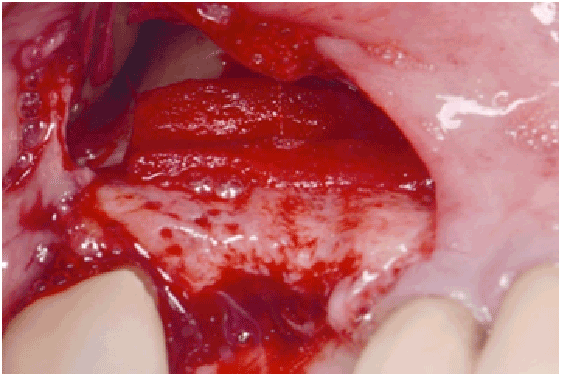
Figure 1f: Absorbable collagen membrane (CollaTape®) positioned between the wall of the sinus and the perforated sinus membrane
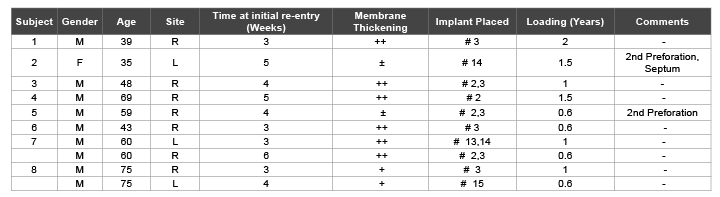
Table 1: Clinical outcomes of re-entry sinus augmentation procedures
++: Membrane thickened and already detached from the sinus walls at re-entry
+: Membrane thickened and attached to the sinus wall at re-entry
±: Re-perforation at re-entry
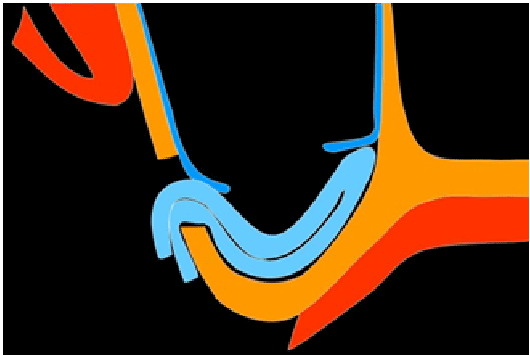
Figure 1g: Diagram of the collagen membrane placement, frontal view
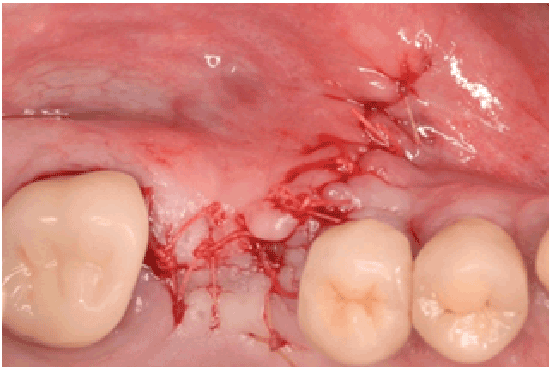
Figure 1h: Primary closure of the flap, tension free achieved (Vicryl 4-0)
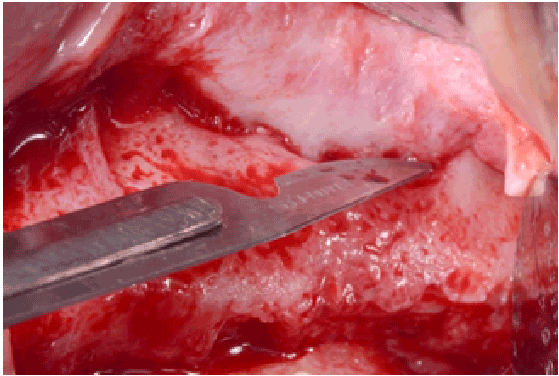
Figure 2a: Partial thickness flap above the previous bony window at re-entry
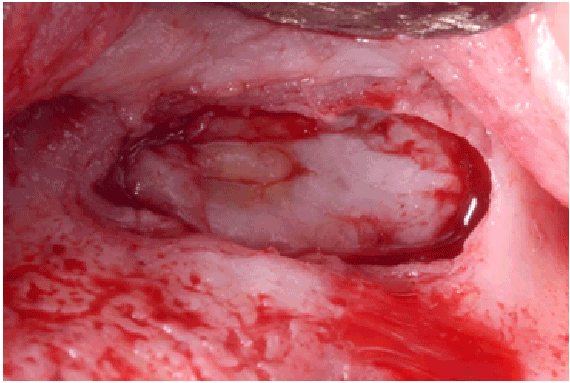
Figure 2b: New thick sinus membrane dissected
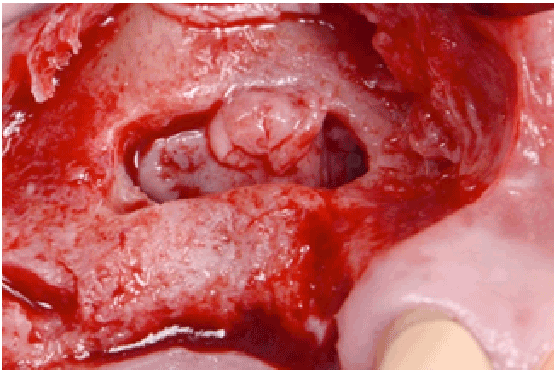
Figure 2c: Elevation of the new thick sinus membrane
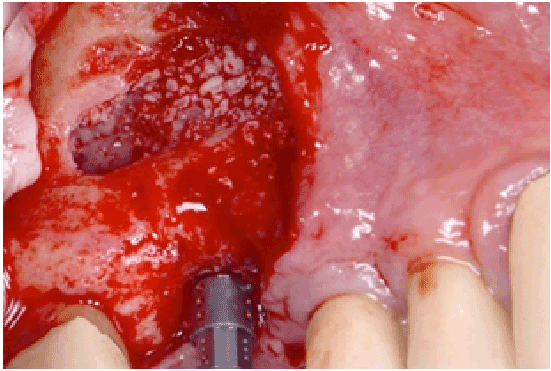
Figure 2d: Bone grafting and implant placement
Eight patients with a total of twelve sinuses had been treated for large perforation following the previously describe protocol. Implants were placed simultaneously for all patients during the re-entry LASFE, representing a total of 15 implants. Clinical assessment of the healed membranes revealed a complete closure and significant thickening in 80% of the cases. Although the remaining 20% showed small reluctant perforations, an augmentation of the membrane thickness was still recorded. Successful LASFE were completed in those cases with an extension of the membrane elevation leading to a collapse of the membrane and thus a closure of the perforations (Figure 3).
Various authors have classified sinus membrane perforation. Fugazzotto and Vlassis [12] introduced a five-class classification based on the location and extent of the perforation. In 2007 Hernandez et al. proposed another classification focused on the perforation diameter [11]. They divided perforations in 3 classes, small (<5 mm), medium (5-10 mm) and large (>10 mm). Large perforations appeared to be the most challenging; therefore, several techniques have been described in the literature to manage them. Abortion of the surgery without any further manipulation of the wounded membrane is one of the options described [13]. On the other hand some authors proposed a repair protocol of the membrane at the time of the perforation [14,15]. However these protocols might increase the length of the procedure, as sinuses membranes are difficult to handle due to their low thickness. In this case series, the technique consisted in a two-stage approach. The purpose of this protocol is to simplify the management of perforated membranes (>10 mm) and to ensure their reparation before grafting. Once a large perforation occurred, decision was made to stop further elevation of the membrane. After a 3 to 6 weeks delay, completion of the SAP was achieved during re-entry surgery. This time frame has been reported as the required period for the soft tissue to heal [16-18].
Stabilization and maturation of the blood clot are critical during the healing of the soft tissue [19]. Therefore, the use of CollaTape® (Integra LifeSciences Corp., Plainsboro, NJ) is meant to enhance those early healing phases. The collagen sponge acts as a space maintainer and provides a 3D collagen scaffold allowing cells recruitment through the wounded area [20,21]. A characteristic connective tissue will then result from these cells activity [16,18,22]. Meanwhile, absorption of the Collatape will occur within 10 to 14 days according to the manufacturer. An uneventful healing was reported as collagen presents low antigenicity and high biocompatibility.
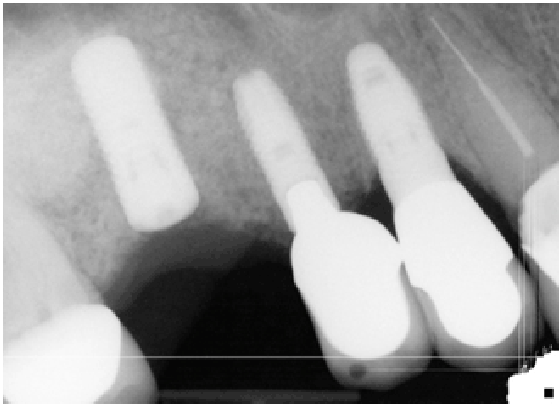
Figure 3: Radiographic image of surgery
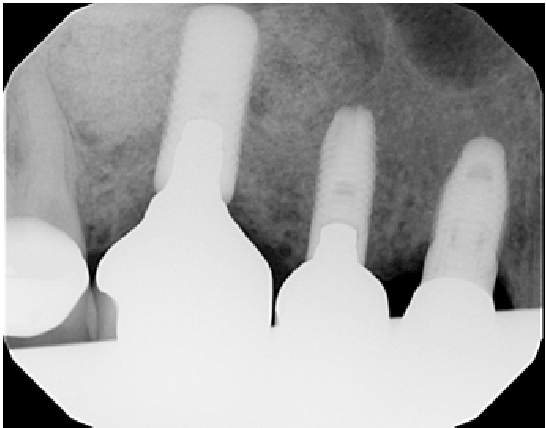
Figure 4: Radiographic image of 1 year follow-up
Thickening of the membrane has been observed in all subjects from the current case series. This observation seems to be related to the scaffold property of the collagen sponge. Similar findings were previously described by Ahn et al in [23]. In their study, large perforations were treated with the use of CollaPlug® followed by abortion of the procedure. Re-entry at 6 months to complete LASFE showed a thicker SM in the window area and an absence of adhesion between the SM and the sinus floor, allowing therefore ease on achieving the LASFE.
In order to increase ease and reliability and also reduce time on LASFE, redesign of the bony window should be performed at the time of the first surgery. The window should be extended in consideration of critical anatomical obstacles to gain access to septa and if needed remove them while avoiding artery. A recent literature review reported a presence of septa in 28.6% of 8,923 sinuses investigated [23]. Furthermore, presence of the antral artery from 47% to 100% was documented. Although 53% of the artery showed a diameter <1 mm, a wounded artery might increase the bleeding on the area and complicate the procedure. Hence such frequent anatomical obstacles should not be neglected.
After 2 years follow-up (Figure 4), this case series maintained a100% success rate. Thickening of the SM lead to an easier procedure and reduce the re-entry surgery length. Within the limitations of this case series, the presented protocol appeared to be a simple and reliable alternative to treat large SM perforation. However, more clinical studies with a greater number of cases and histologic results are needed to confirm these clinical results.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Dagba AS, Mourlaas J, Ochoa Durand D, Suzuki T, Cho SC, et al. (2015) A Novel Approach to Treat Large Schneiderian Membrane Perforation -A Case Series. Int J Dent Oral Health 1(5): doi http:// dx.doi.org/10.16966/2378-7090.137
Copyright: © 2015 Dagba AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access