
Full Text
Shreya Shetty* Bebika Thoudam Aditi Bose
Department of Periodontics, Bangalore Institute of Dental Sciences & Postgraduate Research, Hosur Road, Bangalore – 560029, Karnataka, State, India*Corresponding author:Dr. Shreya Shetty, Department of Periodontics, Bangalore Institute of Dental Sciences & Postgraduate Research, Hosur Road, Bangalore – 560029, Karnataka, State, India, Fax: +918041506025; E-mail: drshreyak@gmail.com
Abstract
Background and Objectives
Herbal dentifrices are fast gaining popularity in most parts of Asia and Europe. Although effects of dentifrices on pathogenic plaque microorganisms has been documented, little or no evidence exists with regard to their effects on the useful microflora, which help to keep the pathogenic strains at bay. The present study was attempted to evaluate and compare these effects of herbal and conventional dentifrice.
Methods
60 student participants in the age group of 18 to 28 years with good oral hygiene (OHI score = 0) were randomly divided into two groups, test and control. The test group used herbal dentifrice and the control group, a regular dentifrice. Plaque and saliva samples were collected at baseline, immediately after brushing, 12 hours after brushing, at 1 week and at 1 month, and cultured. The Streptococcus mitis and Lactobacillus counts along with total microbial counts were estimated at the various time intervals.
Results
Day 1 showed no significant difference between test and control groups with regard to Streptococcus mitis and Lactobacillus counts as well as total CFU (P>0.05), but at one week control group showed significant increase in streptococcus count in plaque and saliva(P>0.01). Whereas, the test group showed significant increase in L. bacillus and total CFU count in saliva, 12 hrs after brushing, when evaluated at one month (p>0.01).
Conclusion
Herbal dentifrices may be slightly more effective in preservation of beneficial oral microflora when compared to regular dentifrices, although both are equally effective in inhibiting the pathogenic microorganisms.
Keywords
Dentifrice; Herbal agents; Beneficial bacteria; Pathogenic bacteria; Plaque
Introduction
Dental plaque is the primary etiologic factor in gingival inflammation, the condition that is followed by chronic periodontitis, due to the apical extension of supragingival plaque into subgingival area [1]. The oral microflora is diverse which includes species such as Neisseria, Staphylococcus, S. pneumoniae, Porphyromonas gingivalis, Diphtheriod, Fusobacteria and Haemophilus [2]. Useful commensals include Streptococcus sanguis, Streptococcus mitis, Streptococcus salivarius, Lactococcus lactis, Lactobacillus casei, Actinomyces naeslundii and Actinomyces viscosus and the harmful ones include Prevotella intermedia, P. gingivalis and Actinobacillus actinomycetemcomitans, T. forsythia, T. Denticola [2]. The most important characteristics of commensal bacteria are their ability to prevent the activation of the host immune system. One proposed mechanism is the development of tolerance, which includes the generation of suppressive T-lymphocytes and the presence of inhibitory cytokines, mainly transforming growth factor-beta and interleukin (IL)- 10 which differentiates commensal from pathogenic organisms based on the former contributing to tolerance and the latter inducing powerful adaptive immune responses. Thus chemical plaque control should be aimed not only at selective elimination of pathogenic microbes but also effective preservation of the beneficial microflora. A dentifrice efficiently reduces harmful oral bacterial flora thus contributing effectively to oral health. Elimination of microbial dental plaque biofilm prevents gingivitis, periodontitis, and dental caries. Studies have shown that dental plaque can be controlled by physical removal, use of dentifrices and mouthwashes [3,4]. However, a conventional toothpaste has its limitations; such as using fluoride in the right concentration (a very low concentration is needed), at the right place (the oral cavity) and at the right time, (available continuously in a mouth where the disease is happening) which favors its effect to control caries. Similarly, triclosan, a common ingredient in toothpastes, has been suggested to have carcinogenic, mutagenic, or teratogenic effects [5]. Because of their widespread use over many years, the issue of the emergence of bacterial resistance has been raised, particularly in relation to the possible development of concomitant resistance to clinically important antimicrobials [6].
On the other hand, herbal dentifrices have the advantages of having no dye or artificial flavouring and possessing antibacterial, antifungal and antimicrobial activity. Studies suggest that Neem extract in herbal toothpaste is appropriate for treating gingivitis and oral infections as it inhibits the formation of plaque and the growth of bacteria [7]. To date, an insufficient amount of clinical research on herbal- based dentifrices has been reported. Only a limited number of in vivo studies on herbal dentifrices have been published suggesting that they may be equivalent to conventional dentifrices in reducing plaque. Although there are positive reports of the effects of dentifrices on the pathogenic plaque microflora, there are limited or no reports whatsoever of the effects of dentifrices on beneficial bacteria in the oral cavity. Thus the present study was aimed to evaluate the antimicrobial effects of herbal and commercially available dentifrice; and also their effects on the beneficial bacterial species, Streptococcus mitis and Lactobacillus count levels in saliva.
Materials and Methods
Following approval from the Ethical Committee, Bangalore Institute of Dental Sciences & Post graduate Research, this pilot study was conducted on students of the Institute in the age group of 18 to 28 years. Sixty patients who met the inclusion criteria, were randomly and equally allotted to the test or control groups; each group consisting of 15 males and 15 females thereby eliminating both age and gender bias.
The inclusion criteria for selection:
- Patients with good oral hygiene (OHI score = 0).
- The exclusion criteria for selection
- Subject with any systemic disease.
- Subject with history of any drug intake including antibiotics, analgesics or any other drugs three months prior to study
- A recent history or presence of any acute or chronic infections.
- Physically or mentally challenged patient.
- Presence of fixed orthodontic appliances.
- Patients who are smokers/paan/tobacco/betelnut users.
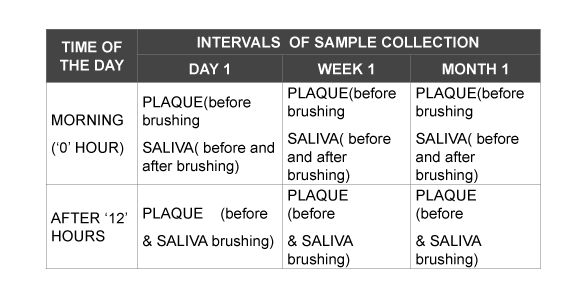
To avoid carry – over effects, a wash out period of two weeks was given prior to the baseline count. Randomisation was done by drawing lots and allotting the patients randomly into test and control groups. The test group was administered herbal dentrifice (Himalaya Drug Company, Bangalore) and the control group Pepsodent G (Hindustan Unilever Limited, Mumbai). All the participants were given oral hygiene instructions and instructed to brush using the given dentifrices twice daily (every 12 hours). Following usage of dentifrice, plaque and saliva samples were collected from the patients of both groups as follows: The collected samples were cultured and colonies of Streptococcus mitis and Lactobacillus counts were compared.

Saliva collection
Unstimulated saliva was collected by expectorating in a sterile eppendorf tube.
Plaque collection
Plaque was collected from the lingual and buccal surface of molar with the help of curettes and then placed inside the eppendorf tube filled with saline.
Laboratory procedures
All the samples were subjected for sonication for 15 seconds and were serially diluted using phosphate buffered saline (PBS) 10 fold dilutions were carried out up to 107. The diluted samples were then vortexed using a cyclomixer. The required plate count agar (Himedia M091) was prepared as per the manufacturer’s instructions. 100 µl of each diluted sample was transferred to sterile disposable petridishes (90 mm). The prepared plate count agar was kept in molten state and maintained at 450°C. About 15 to 20 ml was added to the petridishes and allowed to solidify. After solidification the petridishes were inverted and transferred to a bacteriological incubator set at 37°C and incubated for 24 to 48 hrs. The colonies were manually counted using bacteriological colony counter expressed as Cfu/ml
Calculation = No of colonies Volume of the sample added × dilution factor
For specific organisms specific and selective media were used as follows.
Mitis salivarus agar: Himedia M259 was prepared as per manufacturer’s instructions and used for the isolation and enumeration of Streptococcus mitis. Sample processing and plating procedures were similar to that of total aerobic count.
MRS lactobacillus agar: Himedia was prepared as per the manufacturer’s instructions. This is a selective medium for isolation and enumeration of lactobacillus species. Sample processing and plating procedures were similar to that of total aerobic count.
Total microbial count analysis: Plate count agar of Himedia (M091) was used for the enumeration of total aerobic microbial count.
Statistical analysis
The data obtained were analysed using SPSS (statistical package for social sciences) V 15.0 for windows, installable software that enables assessment of data using several statistical functions. Mann-Whitney U test was done to compare test and control groups with respect to S. mitis, Lactobacillus and total CFU counts in plaque and saliva samples at 1 day, 1 week and 1 month.
Results
A total of 60 subjects, 30 males and 30 females, age range 18 to 28 years completed the study. At the end of day 1, there was no significant difference between test and control group with respect to Streptococcus mitis, Lactobacillus and total CFU counts in plaque samples with level of significance being 5% (p>0.05) suggesting that the counts in the plaque samples were similar at ‘0’ and 12 hours (Table 1). Similarly there was no significant difference between test and control group with respect to Streptococcus mitis, L. Bacillus and total CFU counts in the saliva samples on day 1 (p>0.05) again suggesting that the salivary counts did not vary much before, after brushing and 12 hours after brushing (Table 2).
On the other hand, at the end of 1 week, there was a significant increase in streptococcus counts in both the plaque and saliva samples in the control group before brushing (p>0.01) (Table 3) whereas in the test group, there was a significant increase in Lactobacillus counts when compared before brushing and after 12 hours and total CFU counts when compared before and after brushing in the saliva samples only (p>0.01) (Table 4).
Subsequently, at the end of 1 month, the ‘0’ hour plaque sample showed a significant increase in the lactobacillus counts in the test group (p>0.01) (Table 5). Similarly, the before and after brushing saliva samples showed a significant increase in lactobacillus counts in the test group only (p>0.01) (Table 6). In contrast, the 12 hour saliva sample showed a significant increase in the total CFU counts in the control group only (p>0.01) (Table 6).
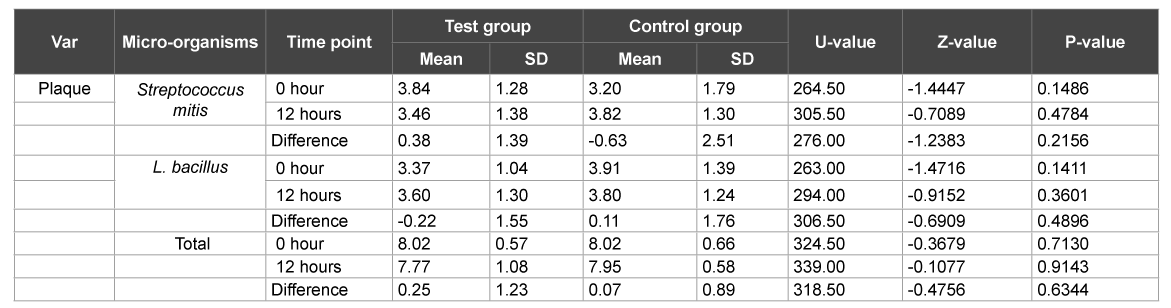
Table 1:Comparison of test and control groups with respect to Streptococcus mitis, L. bacillus and total CFU counts in plaque samples by Mann-Whitney U test at 1 day
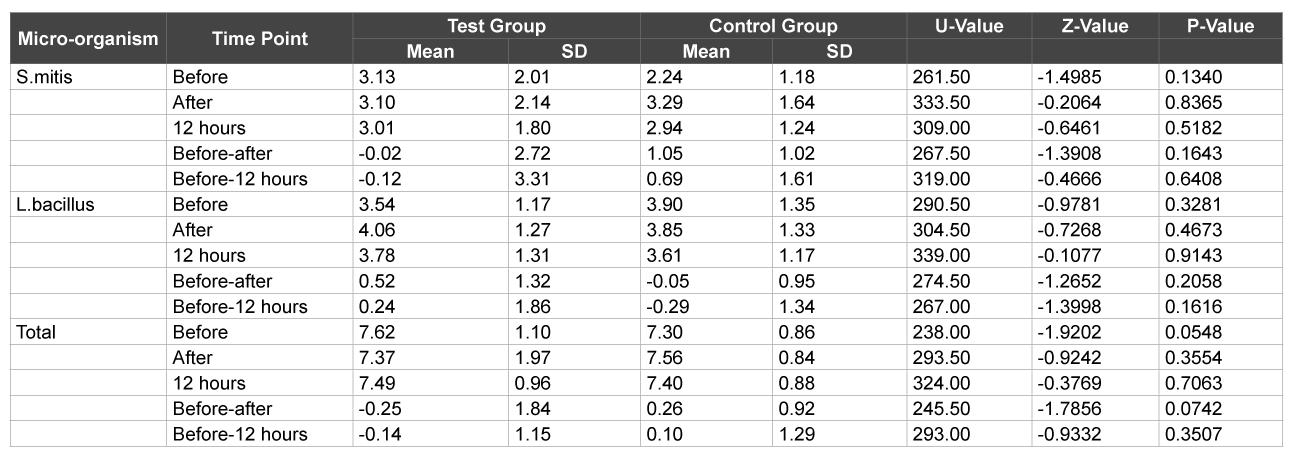
Table 2: Comparison of test & control groups with respect to S. mitis, L. bacillus & total CFU counts in saliva samples at 1 day
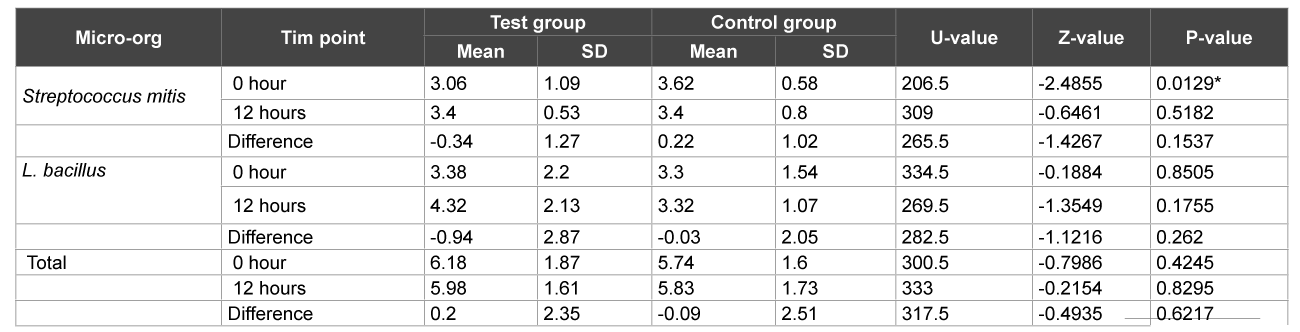
Table 3: Comparison of test and control groups with respect to S. mitis, L. bacillus and total CFU counts in plaque samples at 1 week
Discussion
Most dentifrices available today guarantee complete oral health and complete care for the gingiva and teeth. Herbal dentifrices have been in use for a fairly long period of time and are fast gaining popularity in most parts of Asia and Europe mainly because they are derived naturally and do not possess harsh chemicals present in most commercially available dentifrices.
Antiplaque agents are designed to prevent the formation of the biofilm, and/or remove established biofilm. In contrast, the mode of action of antimicrobial agents involves inhibiting the growth or killing the target bacteria, expressed in terms of their Minimum Inhibitory Concentration (MIC) or Minimum Bactericidal Concentration (MBC), respectively. However, bacteria growing on a surface as a biofilm show reduced sensitivity to killing by antimicrobial agents, especially in older (more mature) biofilms. A major requirement of the formulation, therefore, is to deliver sufficient concentration of the inhibitor in those two minutes of brushing to ensure retention on the dental and mucosal surfaces of the mouth so that the active components can be released over time at levels that will still deliver biological activity. This property of product retention is termed substantivity, and varies markedly among antimicrobial agents [8]. Dentifrices do achieve this goal by favourably reducing the so called disease producing bacteria in plaque [9]. Those which contain triclosan have a purported broad spectrum of antimicrobial activity; and can also reduce inflammation [10]. Sub-lethal levels of triclosan can also inhibit acid production by oral streptococci and protease activity by P. gingivalis. Additive anti-plaque and anti-gingivitis effects were reported when triclosan was combined with a complementary antimicrobial agent such as zinc [10]. Surfactants such as sodium lauryl sulfate can disrupt biofilm structure, damage cell membranes and kill bacteria (when present at high concentrations) and inhibit enzymes (at lower concentrations) [11]. Although many antimicrobial agents used in oral care products are described as being broad spectrum, under the conditions of use in the mouth (variable concentration/short contact times) they probably have a favourably selective mode of action in which they mainly inhibit the growth and metabolism of organisms implicated in disease while leaving those associated with oral health relatively unaffected [12,13]. Plaque also contains certain bacteria which play a very important role in periodontal health by keeping the pathogenic strains at bay [14-16]. Commensal bacteria like Streptococcus salivarius and Lactococcus lactis are few among these. S.salivarius has been found to inhibit Staphylococcus aureus infections and L. lactis has been shown to produce nicin which is a bacteriocin, or a toxin which inhibits similar bacteria. Nicin is also used to prevent bacterial pathogens in the food industry [17]. S. salivarius, along with other oral commensals, plays an important role in the suppression of pathogenic yeast like Candida albicans in human buccal epithelial cells [18]. In addition, S. salivarius strain K12 is known to produce bacteriocins that make it useful in inhibiting Streptococcus pyogenes and other bacteria thought to cause halitosis [19]. It also expresses urease in low pH conditions in the mouth, resulting in the hydrolysis of urea into carbon dioxide and ammonia and ultimately a raised pH. Elevation of the pH creates an environment that is not conducive for acidogenic bacteria like S. mutans [20]. Lactobacillus casei is another common oral microbe which has been shown to support healthy cellular function and immune system response while promoting healthy bacteria and modifying harmful bacterial activities [21]. A study by Laura Walters [22] suggested that the use of xylitol which is a component of dentifrices could cause inhibition of L. lactis over time but does not have a detrimental effect on S. salivarius and L. casei
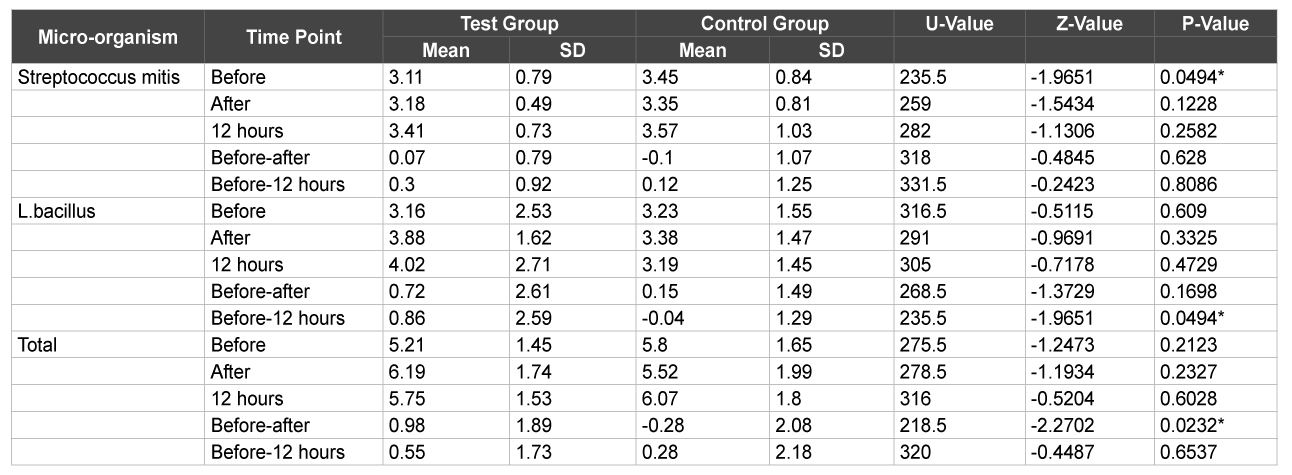
Table 4: Comparison of test & control groups with respect to S. mitis, L. bacillus & total CFU counts in saliva samples at 1 week
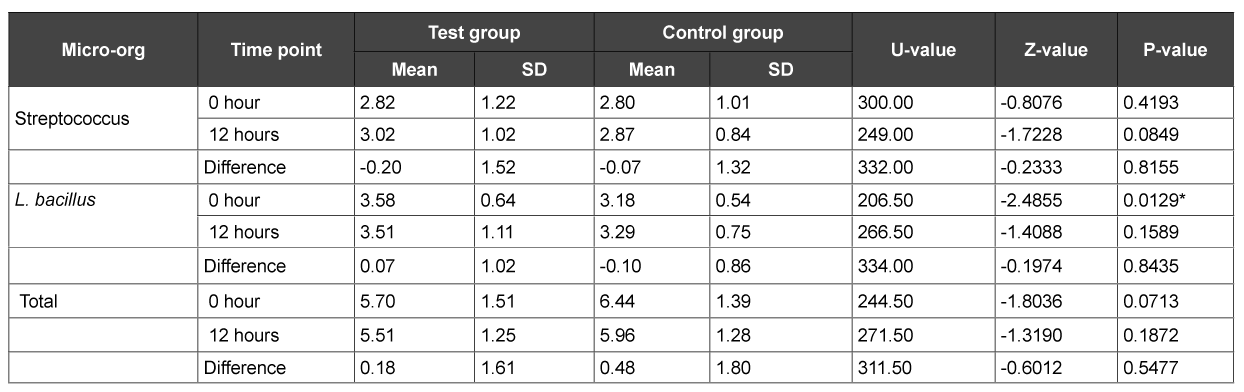
Table 5: Comparison of test and control groups with respect to S. mitis, L. bacillus and total CFU counts in plaque samples at 1 month
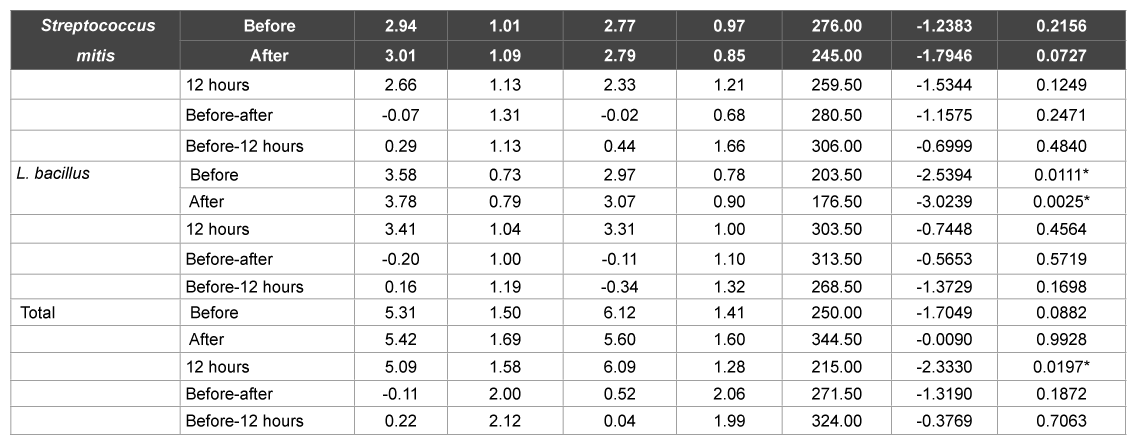
Table 6: Comparison of test and control groups with respect to S. mitis, L. bacillus and total CFU counts in saliva samples at 1 month
To our knowledge, no clinical study has so far assessed the effects of chemical anti-plaque agents on the useful microflora. A lot of research has focussed on the therapeutic effects of various agents on pathogenic plaque. However, the positive or negative effects of these agents on useful microflora are yet to be assessed. The present study was therefore carried out to evaluate the effects of these dentifrices on 2 beneficial bacterial species, Streptococcus mitis and Lactobacillus and on the total microbial counts on periodontally and systemically healthy subjects. It was decided to assess the microbial counts at various time intervals based on the claim made by conventional commercial dentifrices that the mouth can be kept plaque free for 12 hours following their use. Thus plaque and saliva samples were collected and assessed at 3 intervals to evaluate the actual effects of these dentifrices.
A wash out period of 2 weeks was maintained to primarily exclude the residual effects if any, of the previous dentifrice used by the patient. This was done to eliminate any bias and maintain standardisation with regard to the study protocol.
As was evident from the results, no significant difference was found in both the herbal and conventional dentifrice groups in the plaque as well as saliva samples with respect to Streptococcus mitis, Lactobacillus and CFU counts at the various time intervals on day 1 after usage. However, at the end of 1 week, plaque and saliva samples showed significant increase in the Streptococcus mitis counts before brushing in the conventional dentifrice group when compared to the herbal dentifrice group. On the other hand, saliva samples of the herbal dentifrice group showed significantly higher Lactobacillus counts and total CFU counts when compared to the conventional group immediately after brushing and 12 hours after brushing. Similarly, at the end of 1 month, the Lactobacillus counts in plaque and saliva were significantly higher in the herbal dentifrice group than the conventional group with no difference in the streptococcus counts. On the other hand, the total CFU counts in the 12 hour saliva sample were significantly higher in the conventional dentifrice group. These findings clearly indicate a more effective preservation of the oral microflora by the herbal dentifrice when compared to the regular conventional dentifrice. In addition, over an extended period of time, the herbal dentifrice also showed better antimicrobial effects compared to the conventional dentifrice. This antimicrobial activity is in accordance with studies [24-28] where herbal dentifrices were found to be effective against dental plaque microorganisms. Till date, there has been no study to elucidate the effects of dentifrices or any other antiplaque agent on the beneficial commensal bacteria. Tooth brushing with the aid of a dentifrice is a basic tool employed to maintain oral hygiene and therefore, it is imperative to get a broad perspective on its effects on the plaque microflora, either beneficial or otherwise. This is the first study of its kind to assess the same and as is evident, herbal dentifrices proved to be slightly better at preservation of the beneficial bacteria than their conventional counterparts.
Conclusion
Although healthy microflora are effectively preserved by both the conventional and herbal dentifrices as is evident from the lack of statistical significance, the herbal dentifrice turned out to be marginally better; although both dentifrices were equally effective in reducing pathogenic plaque and maintaining oral hygiene. Furthermore, long term studies on a larger cross section of the population need to be carried out and the use of a more sensitive test such as PCR is recommended to assess the antimicrobial activity.
Acknowledgements
The authors wish to thank and acknowledge m/s Himalaya Drug Company, Bangalore, India for funding this project and also the kind services of Dr.Prahlad Patki, former head - medical services and clinical trials, and Dr. Sandeep Varma, Himalaya Drug Company, for their valuable assistance in carrying out this study with regard to providing the drug samples and their laboratory facilities for analysing the biochemical parameters of the study.
References
- Hancock EB (1996) Prevention. Ann Periodontol 1: 223-249.
- Yost C (2005) Types of Bacteria in Your Mouth. [Ref.]
- British Dental Health Foundation, FAQ, Caring for my teeth. [Ref.]
- Collins WJ, Walsh TF (1996) Handbook for dental hygienists. Oxford University Press, UK 272-273. [Ref.]
- Bhargava HN, Leonard PA (1996) Triclosan: applications and safety. Am J Infect Control 24: 209-18. [Ref.]
- Ledder RG, Gilbert P, Willis C, McBain AJ (2006) Effects of chronic triclosan exposure upon the susceptibility of 40 ex-situ environmental and human isolates. J Appl Microbiol 100: 1132–1140. [Ref.]
- Pai MR, Acharya LD, Udupa N (2004) Evaluation of antiplaque activity of Azadirachta indica leaf extract gel - a 6-week clinical study. J Ethnopharmacol 90: 99-103. [Ref.]
- Cummins D (1992) Mechanisms of action of clinically proven antiplaque agents. In: Clinical and Biological Aspects of Dentrifrices. Oxford: Oxford University Press UK 205−28.
- Renvert S, Birkhed D (1995) Comparison between 3 triclosan dentifrices on plaque, gingivitis and salivary microflora. J Clin Periodontol 22: 63- 70. [Ref.]
- Brading MG, Marsh PD (2003) The oral environment: the challenge for antimicrobials in oral care products. Int Dent J 53: 353−362. [Ref.]
- Scheie AA, Petersen FC (2008) Antimicrobials in caries control. In: Dental Caries. The Disease and its Clinical Management, 2nd edn. Oxford: Blackwell Munksgaard 265−77.
- Marsh PD (1992) Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res 71: 1431−1438. [Ref.]
- Bradshaw DJ, Marsh PD,Watson GK, Cummins D (2009) The effects of triclosan and zinc citrate, alone and in combination, on a community of oral bacteria grown in vitro. J Dent Res 72: 25−30. [Ref.]
- George J, Hegde S, Rajesh KS, Kumar A (2009) The efficacy of a herbal-based toothpaste on the control of plaque and gingivitis. Indian J Dent Res 20: 480-482. [Ref.]
- 15 Radafshar G, Mahboob F, Kazemnejad E (2010) A study to assess the plaque inhibitory action of herbal-based toothpaste: A double blind controlled clinical trial. Journal of Medicinal Plants Research 2010; 4(12): 1182-1186.
- Mullally BH, James JA, Coulter WA, Linden GJ (1995) The efficacy of a herbal-based toothpaste on the control of plaque and gingivitis. J Clin Periodontol 22: 686-689. [Ref.]
- Heikkila MP, Saris PEJ (2003) Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol 95: 471–478. [Ref.]
- Nair R, Samaranayake LP (1996) The effect of oral bacteria on candidal adhesion to buccal epithelial cells in vitro. J Dent Res 75: 204. [Ref.]
- Burton JP, Chilcott CN, Moore CJ, Speiser G, Tagg JR (2006) A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol 100: 754-764. [Ref.]
- Yi-Ywan MC, Hall TH, Burne RA (1998) Streptococcus salivarius urease expression: involvement of the phosphoenolpyruvate:sugar phosphotransferase system. FEMS Microbiology Letters 165: 117- 122. [Ref.]
- Aryana, KJ and McGrew P (2007) Quality attributes of yogurt with Lactobacillus casei and various prebiotics. LWT Food Sci Technol 40: 1808-1814. [Ref.]
- Laura Walters (2011) The Effect of Xylitol on the Growth of Three Normal Oral Commensal or Probiotic Bacteria [Ref.]
- Abdulwahab IA (2011) Comparison between the Efficacy of Herbal and Conventional Dentifrices on Established Gingivitis. J Dent Res 8: 57- 63. [Ref.]
- Bellet L, Bellet A (1988) Comparative clinical trials of a European herbal sodium bicarbonate dentifrice and a widely- used dentifrice containing MFP in brace- induced gingivitis. J Clin Dent: A25-A26. [Ref.]
- Emling RC (1988) Double-blind evaluation of the clinical efficacy of an herbal dentifrice against gingivitis and periodontitis. J Clin Dent A27-A29. [Ref.]
- Ozaki F, Pannuti CM, Imbronito AV, Pessotti W, Saraiva L, et al. (2006) Efficacy of a herbal toothpaste on patients with established gingivitis. A randomized controlled trial. Braz Oral Res 20: 172-177. [Ref.]
- Pannuti CM, Mattos JP, Ranoya PN, Jesus AM, Lotufo RFM, et al . (2003) Clinical effect of a herbal dentifrice on the control of plaque and gingivitis. A double-blind study. Pesq Odontol Bras 17: 314-318. [Ref.]
- Yankell SL, Dolan MM, Emling RC (1998) Laboratory evaluations of an herbal sodium bicarbonate dentifrice. J Clin Den A6-A8. [Ref.]
Download Provisional PDF Here
Article Information
Article Type: Review Article
Citation: Shetty S, Thoudam B, Bose A (2015 Comparative Evaluation of the Effect of a Herbal Dentifrice and a Regular Dentifrice on Beneficial Oral Microflora – A Clinico-Microbiologic Study. Int J Dent Oral Health 1(3): doi http://dx.doi.org/10.16966/2378-7090.117
Copyright:© Shetty S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
