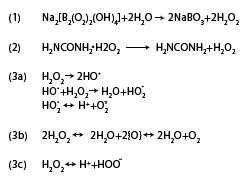Introduction
Tooth discoloration varies in etiology, appearance, localization, severity, and adherence to tooth structure. It may be classified as intrinsic, extrinsic, and a combination of both [1].
Intrinsic discoloration is caused by incorporation of chromatogenic
material into dentin and enamel during odontogenesis or after eruption.
Exposure to high levels of fluoride, tetracycline administration, inherited
developmental disorders, and trauma to the developing tooth may result
in pre-eruptive discoloration. After eruption of the tooth, aging, pulp
necrosis, and iatrogenesis are the main causes of intrinsic discoloration.
Coffee, tea, red wine, carrots, oranges, and tobacco give rise to extrinsic
stain [2]. Wear of the tooth structure, deposition of secondary dentin due
to aging [2] or as a consequence of pulpnecrosis, or as a consequence
of pulp inflammation, and dentin sclerosis affect the light-transmitting
properties of teeth, resulting in a gradual darkening of the teeth.
Scaling and polishing of the teeth remove many extrinsic stains.
For more stubborn extrinsic discoloration and intrinsic stain, various
bleaching techniques may be attempted.
Tooth bleaching can be performed externally, termed night guard vital
bleaching or vital tooth bleaching, or intracoronally in root-filled teeth,
called non-vital tooth bleaching. The aims of the present review article
are to review critically the literature on the biological aspects of tooth
bleaching, including efficacy and side-effects of such treatments.
History of Bleaching
Bleaching of discolored, pulpless teeth was first described in 1864 [3] and
a variety of medicaments such as chloride, sodium hypochlorite, sodium
perborate, and hydrogen peroxide has been used, alone, in combination,
and with and without heat activation [4]. The “walking bleach” technique
that was introduced in 1961 involved placement of a mixture of sodium
perborate and water into the pulp chamber that was sealed off between
the patient’s visits to the clinician [5]. The method was later modified and
water replaced by 30-35% hydrogen peroxide, to improve the whitening
effect [6]. The observation that carbamide peroxide caused lightening of
the teeth was made in the late 1960s by an orthodontist who had prescribed
an antiseptic containing 10% carbamide peroxide to be used in a tray for
the treatment of gingivitis [7]. The observation was communicated to
other colleagues and must be regarded as the beginning of the night guard
bleaching era. More than 20 years later, the method describing the use
of 10% carbamide peroxide in a mouth guard to be worn overnight for
lightening tooth color was published [8]. The mechanistic details of the 3
preferred bleaching methods are summarized in the Figure 1.
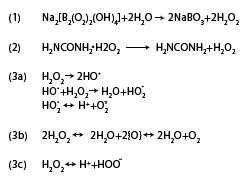
Figure 1: The formation of hydrogen peroxide from sodium perborate
(Eq. 1) (Hägg G [91]) and from carbamide peroxide (Eq. 2) ( Budavari
et al. [92]). Hydrogen peroxide forms free radicals like hydroxyl and
perhydroxyl radicals, and superoxide anions (Eq. 3a)(Gregus and
Klaassen, 1995), reactive oxygen molecules that are unstable and
transformed to oxygen (Eq. 3b) and hydrogen peroxide anions (Eq. 3c)
(Cotton FA, Wilkinson G [93]).
The pH of Tooth-Whitening Products
Despite various potential toxicological side effects [9-11], peroxides
have been used for many years to treat periodontal diseases [12,13].
Peroxides, usually in the form of hydrogen peroxide or carbamide
peroxide, are also the active ingredient in most tooth-whitening agents.
The safety, effectiveness and various side effects of these products on the
intraoral structures have been investigated, and some products have been
accepted by the American Dental Association for whitening teeth [14-18).
However, some bleaching products have been reported to have a pH as
low as 4.0, while others have been reported to have a pH of 7.5 [19]. It has
been reported that the greater the peroxide concentration, the more acidic
the pH of the bleaching product [20]. Some in-office bleaching products
that contain 35% hydrogen peroxide may have a low pH.
Subjecting the teeth and oral tissues to a low or high pH for an extended
period of time may cause adverse side effects. When the pH falls below
5.2, enamel demineralization [21] and root resorption have been reported
[20,22]. Recent research to investigate the effects of pH on enamel suggests
that low pH and high acid concentrations cause enamel erosion [23,24].
Interestingly, adding small amounts of calcium to acidic solutions may
decrease enamel loss by up to 50% [24,25].
Genotoxicity and Carcinogenicity of Bleaching Agents
The genotoxicity of hydrogen peroxide and of tooth whiteners
containing carbamide peroxide has been evaluated [26]. The consensus
arising from these evaluations was that direct contact with hydrogen
peroxide induced genotoxic effects in bacteria and cultured cells. When
hydrogen peroxide was administered to bacteria or cultured cells in
the presence of catalase or other metabolizing enzymes, the effect
was reduced or abolished. Testing of hydrogen peroxide for systemic
genotoxic effects in animals revealed no evidence of in vivo mutagenicity.
Since hydroxy radicals perhydroxyl ions, and superoxide anions formed
from hydro-gen peroxide are capable of attacking DNA, the genotoxic
potential of hydrogen peroxide is dependent on the accessibility of
free radicals to target DNA. This may explain why hydrogen peroxide
induces genotoxicity in the presence of metabolizing enzymes neither in
vitro nor in vivo. Tooth whiteners containing carbamide peroxide were
mutagenic in certain bacterial strains and non-mutagenic in the presence
of additional activating enzymes. Several in vivo studies addressing the
formation of micronuclei in bone marrow cells and sister chromatide
exchange after exposure to carbamide-peroxide-containing products
revealed no genotoxic effects.
Based on the aforementioned studies, hydrogen peroxide was shown to have a weak local carcinogenic-inducing potential. The mechanism is unclear, but a genotoxic action cannot be excluded, since free radicals
formed from hydrogen peroxide are capable of attacking DNA. Several studies of DMBA carcinogenesis in mice skin and hamster cheek pouch indicate thathydrogen peroxide may act as a tumor-promoter [10,27]. The International Agency for Research on Cancer (IARC) concluded that there is limited evidence in experimental animals and inadequate evidence in humans for the carcinogenicity of hydrogen peroxide and classified the chemical into Group 3: Unclassifiable as to carcinogenicity
to humans [28]. It appears unlikely that oral health products containing or releasing hydrogen peroxide up to 3.6% H2 O2 will enhance cancer risk in individuals except in those who have an increased risk of oral cancer
due to tobacco use, alcohol abuse, or genetic pre-disposition [29]. To evaluate higher concentrations of hydrogen peroxide was not the task of the committee.
Free Radical Formation, Antioxidants and Relevance in Health:
Generation of free radicals in living systems
The formation of free radicals can be from endogenous or exogenous
origin. Endogenous free radicals are continuously produced during
the normal metabolic processes in the body’s normal use of oxygen, an
element indispensable for life. The most important of these by-products
consists of reactive oxygen species (ROS) as well as reactive nitrogen
species (RNS) that result from the cellular redox process [30]. During
cell respiration, oxidative phosphorylation takes place in the electron
transport chain during which most of the oxygen is consumed. During
the process, energy is trapped in the form of ATP and oxygen is converted
to water. However, during incomplete reduction of O2 by a single electron addition, the superoxide radical O2 •¯ is formed [31]. Superoxide is regarded as the primary ROS, and once formed this highly reactive species
can start several enzymatic reactions responsible for the formation of different ROS such as H2 O2 , the highly reactive hydroxyl radical (OH•)¯, as well as reactive nitrogen species (RNS) such as nitric oxide (NO•),
peroxynitrite (ONOOˉ) and hypochlorous acid (HOCl) [32]. Nitric
oxide is a RNS produced by enzymes which metabolise arginine, and is
used as signaling molecules in a number of physiological processes such
as neurotransmission, blood pressure regulation, defence mechanisms,
smooth muscle relaxation and immune regulation [32]. Exogenous origins
of free radicals include heat, trauma, ultrasound, ultraviolet light, ozone,
smoking, exhaust fumes, radiation, dental materials, infection, excessive
exercise and therapeutic drugs [33].
Antioxidants
Antioxidants are substances that are able to reduce the free radical
concentrations in cells and the body. They also react at different
stages of the free radical formation. They can be preventive, such as
superoxide dismutase or interceptive, such as vitamin C. Furthermore;
the antioxidant defense systems of the human body can be classified
into two groups, the enzymatic- and the non-enzymatic defence system.
Most of the antioxidants present in the cell such as superoxide dismutase,
glutathione peroxidase, catalase, glutathione reductase and thioredoxin
are endogenous enzymatic systems, and produced within the cell [34]. Superoxide dismutase is an important endogenous antioxidant, and the first line of defence against free radicals. It converts superoxide radicals
to H2 O2 . Then, the glutathione peroxidase and the catalase present in the
cytoplasm of the cells will remove the H2 O2 produced by reducing it to H2 O [35].
Vitamin E and vitamin C, together with other exogenous chemicals
such as carotenoids and flavonoids, are essential nutrients and nonenzymatic
antioxidant scavengers obtained from the normal human diet
[36]. They mostly act as an interceptive radical chain, breaking reactions
by trapping peroxyl and other reactive radicals [35].
Non-enzymatic antioxidants can also be metabolic antioxidants.
Metabolic antioxidants are the endogenous antioxidants produced by
metabolism in the body and include lipoid acid, glutathione, L-ariginine,
co-enzyme Q10, melatonin, uric acid, bilirubin, metal-chelating proteins,
etc [35].
Oxidative stress
Although the harmful effects of free radicals in biological systems
were discovered about half a century ago, the importance of free radicals
and antioxidants, and the therapeutic potential of the latter in health and
disease, only became clear in recent years [35]. Usually, low or moderate
concentrations of ROS and RNS form part of the development process
of cellular structures, and the host cellular defense mechanisms such as
the phagocytic destruction of bacteria [35]. Normally, there is a balance
between the formation and removal of these free radicals. However,
when this balance is shifted towards overproduction of free radicals or
the removal of free radicals is diminished as a result of a shortage of
antioxidants, oxidative stress develops. Because these free radicals have
an affinity for nucleic acids, proteins and lipids, they play a pivotal role
under conditions of oxidative stress in the development of a number of
chronic and degenerative diseases [35]. Recently, it has been claimed
that oxidative stress in saliva may play an important role in the onset of
periodontal diseases [35]. Furthermore the oxidative stress in patients
with periodontal disease could lead to the development of cardiovascular
disease [35].
Antioxidants counteract oxidative stress and thereby lower the risk
of the chronic and degenerative diseases [35]. Human saliva is rich in
antioxidants such as uric acid, albumin, vitamin C and enzymes, which
can be used as biomarkers in the measurement of oxidative stress in the
oral cavity [37]. Since oxidative stress in the oral cavity can also be linked
to certain systemic diseases [38] measurement of these biomarkers in
saliva may provide an accurate indicator of oxidative stress in the body
[38].
Advantages of Free Radicals in the Cell
Although free radicals can be very harmful to the body, they are also
vital to human life. At low concentrations they are essential for normal
physiological functions where they help to maintain homeostasis at the
cellular level and play an important role as signaling molecules. Some
of the important physiological functions include the generation of ATP
used for energy transfer during oxidative phosphorylation, phagocytic
destruction of invading pathogens, the killing of cancer cells by cytotoxic
lymphocytes, apoptosis of defective cells, redox regulated production
of NO used in the regulation of vascular tone, redox regulation of cell
adhesion and other regulatory functions [37].
Oral Diseases, Oxidative Stress and the Role of Antioxidant Defenses in the Oral Cavity
The oral cavity is lined with delicate mucus membranes that allow
rapidabsorption of harmful chemicals present in food, drinks, tobacco
products and dental materials across its surface. The oral tissues are also
vulnerable to cell damage through trauma, bacterial onslaught and other
disease-causing agents. The oral cavity is therefore, uniquely susceptible to
oxidative stress that can be responsible for a number of oral diseases such
as periodontitis [37], aphthous ulcers [37,38] lichen planus [38] and oral
sub-mucous fibrosis [39] some of which may develop into oral cancers
[40].
Oral lesions, whether they are infected or not, will always trigger
immune responses. During the resulting inflammatory processes, there
is usually an over-production of ROS by the leukocytes promoting
cytotoxicity, and this may initiate and/or amplify inflammation [41,42].
The ROS and RNS produced may initiate free radical chain reactions,
which mediate tissue damage through DNA damage, lipid peroxidation,
protein damage, oxidation of antiprotease and the stimulation of proinflammatory
cytokine release [33]. Periodontitis, one of the most
common oral infections induced by bacteria and bacterial products and
characterized by inflammatory destruction of tooth supporting connective
tissues and alveolar bone, is a good example of this process [41,42].
Antioxidants can remove the harmful effects of these free radicals.
Saliva is a natural defence against bacteria and other substances pernicious
to the oral cavity, and the antioxidants present in saliva are among the
most important elements that aid in protection against these diseases.
Antioxidants present in saliva include uric acid, albumin, ascorbic
acid, glutathione and enzymes such as transferrin, lactoferrin and
ceruloplasmin [43]. While uric acid is the main antioxidant present at high
concentrations in saliva, the role of melatonin as a potent antioxidant, and
its role during oxidative stress in the oral cavity, became of importance
recently [44,45]. Melatonin is formed in the pineal gland and discharged
in blood from where it is released into the saliva via the salivary glands.
Melatonin has strong antioxidant potential and immunomodulatory, antiinflammatory,
and anticancer properties. Melatonin may interfere in the
function of osteoclasts and thereby inhibit bone resorption as a result of
periodontal disease and dental implants. In addition, melatonin decreases
cytotoxic and genotoxic effects of methacrylate monomers used in dental
materials [44,45].
Adverse Effects of Bleaching
Effect of bleaching and hydrogen peroxide on pulp
Studies have been conducted to examine the penetration of hydrogen
peroxide and carbamide peroxide into the pulp chambers of teeth.
Human and canine studies showed that both low (10%) and high (35%)
concentrations of bleaching agents readily penetrate the pulp chamber
[46-49]. Cohen applied 35% hydrogen peroxide and heat for 30-minute
sessions to human teeth due to be removed for orthodontic treatment [47].
Varying degrees of sensitivity, lasting from 24–48 hours, were reported
by 78% of the subjects. Histological findings in both the experimental
and control groups showed that, except for moderate vasodilation and
aspiration of odontoblast nuclei into the dental tubules, all pulps were
normal. There were no histological findings to explain the sensitivity
experienced by the subjects. A possible explanation may be that pressure
builds in the pulp chamber as a result of the heat applied, causing the
sensation of pain. The sensitivity, moderate vasodilation and aspiration
of odontoblasts into the dental tubules appeared to be reversible in most
cases [46-49].
Although clinical observations and scientific literature report shortterm,
minimal hypersensitivity to in-office and at-home bleaching
treatments, there have been studies published that raise concerns about
possible harmful effects of some bleaching agents on the pulp [50-54].
Glucose metabolism and protein synthesis, especially collagen synthesis,
are the two most central metabolic processes occurring in the pulp. These
metabolic reactions are catalyzed by enzymes that are sensitive to changes
in environmental conditions [54]. Bowles and Thompson examined
combined effects of heat and hydrogen peroxide on pulpal enzymes and
found that most of the enzymes were relatively resistant to the effects of
heat up to 50°C [52]. However, nearly every enzyme tested was inhibited to
some degree by hydrogen peroxide. At concentrations as low as 5% some
enzymes were completely inactivated. Results indicated that a combination
of heat and hydrogen peroxide might increase the permeability of the
pulp and potentiate the effects of hydrogen peroxide on the pulp. While
the pulp appears to be quite resilient, there is concern for patients who
may apply bleaching agents for longer periods of time or more frequently
than recommended in order to hasten the achievement of whiter teeth.
The long-term effects of frequent or prolonged use of bleaching agents
on pulps are unknown [51-54]. The reasons for tooth sensitivity during
vital tooth bleaching are not clear. Studies are inconclusive regarding the
pulpal considerations of vital tooth bleaching. What is clear, however,
is that case selection is critical. Considerations prior to initiating tooth
whitening procedures should include assessment of the condition of
existing restorations, cervical erosion, enamel cracks, and the estimated
duration and repetition of bleaching required to obtain and maintain the
desired effect [55].
Adverse effects on hard tooth surfaces
Cervical root resorption is an inflammatory-mediated external
resorption of the root, which can be seen after trauma and following
intracoronal bleaching [56].
A high concentration of hydrogen peroxide in combination with
heating seemed to promote cervical root resorption [56,57], in line
with observations made in animal experiments [58-60]. The underlying
mechanism for this effect is unclear, but it has been suggested that the
bleaching agent reaches the periodontal tissue through the dentinal
tubules and initiates an inflammatory reaction [61]. It has also been
speculated that the peroxide, by diffusing through the dentinal tubules,
denatures the dentin, which then becomes an immunologically different
tissue and is attacked as a foreign body [62]. Frequently, the resorption
was diagnosed several years after the bleaching [56,62]. In vitro studies
using extracted teeth showed that hydrogen peroxide placed in the
pulp chamber penetrated the dentin [63] and that heat increased the
penetration [64]. The penetration has been found, in vitro, to be higher in
teeth with cervical defects of the cementum [64,65], and that may enhance
the effects of hydrogen peroxide following repeated exposures. Based on
the cited literature, the use of a thermo-catalytic bleaching procedure in
teeth with cervical defects of the cementum constitutes a risk factor for
the development of cervical resorption. In addition, efficacy studies have
shown that 30% hydrogen peroxide was not essential to the attainment of
an acceptable treatment outcome.
Tooth crown fracture has also been observed after intra-coronal
bleaching [66] most probably due to extensive removal of the intra-coronal
dentin. In addition, intra-coronal bleaching with 30% hydrogen peroxide
has been found to reduce the micro-hardness of dentin and enamel [67]
and weaken the mechanical properties of the dentin [68].
Local side-effects
Tooth sensitivity is a common side-effect of external tooth bleaching
[69]. Tooth sensitivity normally persists for up to 4 days after the cessation
of bleaching treatment [47,69,70].
The mechanisms that would account for the tooth sensitivity after
external tooth bleaching have not yet been fully established. In vitro
experiments have shown that peroxide penetrated enamel and dentin and
entered the pulp chamber [71] and that the penetration of restored teeth
was higher than that of intact teeth [72]. The amount of peroxide detected
in the pulp chamber was related to the concentration of hydrogen peroxide
in the preparations applied [72], and also varied among different brands
of bleaching agents with the same declared concentration of carbamide
peroxide [71]. The concentration of peroxide in the pulp chamber was
not determined in the above studies, and the clinical significance of the
findings is therefore unclear [73].
Tooth sensitivity was also a common symptom in patients who had
not bleached their teeth, and their symptom was correlated with gingival
recession [74]. Patients with a previous history of tooth sensitivity may
thus have a higher risk for such an adverse effect from external tooth
bleaching, and this should be taken into account before treatment begins.
Mucosal irritation
A high concentration of hydrogen peroxide (from 30 to 35%) is caustic to mucous membranes and may cause burns and bleaching of the gingiva. In animal experiments, exposure of the gingiva to 1% H2 O2
for 6 to 48 hrs resulted in epithelial damage and acute inflammation in the sub-epithelial
connective tissue [75]. It is therefore advisable that the tray be designed to
prevent gingival exposure by the use of a firm tray that has contact with
solely the teeth. In this respect, the newly introduced bleaching strips may
be unfavourable, since the bleaching gel will come into contact with the
gingiva.
Alteration of enamel surface
Morphological alteration of the enamel following tooth bleaching
has been addressed in several in vitro studies [76]. Compared with
the untreated control surfaces, the enamel surface exposed to the
bleaching agents underwent slight morphologic alterations [77]. A high
concentration of carbamide peroxide was detrimental to enamel surface
integrity, but the damage was less than that seen after phosphoric acid
etch [78]. A clinical implication of these findings may be that the teeth
are more susceptible to extrinsic discoloration after bleaching due to
increased surface roughness.
Effects on restorations
Data from laboratory studies documented increased mercury release
from dental amalgams exposed to carbamide peroxide solutions for
periods ranging from 8 hrs to 14-28 days [79,80]. The amount of mercury
released varied with type of amalgam and type of bleaching agent and
ranged from 4 times to 30 times higher than in saline controls. It has been
suggested that bleaching may increase the solubility of glass-ionomer and
other cements [81]. Furthermore, the bond strength between enamel and
resin-based fillings was reduced in the first 24 hrs after bleaching [82].
After 24 hrs, there was no difference in the strengths of dental composite
resin cement bonds to bleached and non-bleached enamel [83].
Following bleaching, hydrogen peroxide residuals in the enamel
inhibit the polymerization of resin-based materials and thus reduce bond
strength [62]. Therefore, tooth-bleaching agents should not be used prior
to restorative treatment with resin-based materials.
To minimize this inconvenience, the treatment of the whitened dental
structure with antioxidants has been recommended, for example, with
sodium ascorbate (10%), to enable the completion of aesthetic restorations
in shorter periods, as sodium ascorbate removes residual oxygen and
promotes higher adhesiveness to the whitened dental substrate [75,84].
Antioxidants derived from ascorbic acid are used for decreasing the
time interval between dental whitening and definitive restoration, thus
enabling for the restoration procedure to be made with the prospect of
maintaining longevity and durability of the adhesive [85]. However,
application time of antioxidants for reverting the adverse effects on
adhesion to the enamel and the dentine [86] has not been considered as
entirely feasible yet, as it is seen as too lengthy for clinical uses.
Several methods have been proposed to decrease micro leakage due to
bleaching, including removal of the superficial enamel layer, pre-treatment
of the bleached enamel with alcohol, use of adhesives containing organic
solvents, cleansing of the cavity with antioxidants, and a post-bleaching
period ranging from 24 h to 3 weeks [87]. It has been shown that removal
of the superficial enamel layer is ineffective because bonding weakens
both superficially and internally [84].
Several antioxidant agents have been introduced, such as sodium
ascorbate, ascorbic acid, butylhydroxyanisole, catalase, ethanol, acetone,
glutathione, peroxide, α-tocopherol, and sodium bicarbonate, in order
to better control restoration micro leakage [84]; however, only a few of
them were found to be effective. Recently, a biocompatible and neutral
antioxidant, 10% sodium ascorbate, was shown to be able to remove the
residual peroxide and oxygen, so that compromised bonding to bleached
tooth structures, such as dentin or enamel, could be reversed [88].
Furthermore, Moosavi et al. (84) demonstrated that the addition of
surfactant (0.2% Tween®
80) to a sodium ascorbate formulation could
significantly reduce the micro leakage after nonvital bleaching. Only one
study [89] has investigated the use of catalase in improving the composite–
resin bond strength after tooth bleaching. In another study [90], it was
reported that catalase could be used as an adjunct to effectively eliminate
residual hydrogen peroxide from the pulp chamber and the surrounding
periodontal tissues following intra-coronal bleaching of non-vital teeth. In
the present study, we examine the effect of catalase on improving adhesion
between composite resin and externally bleached teeth and on reduction
of micro leakage after external tooth bleaching.
Overt signs of hydrogen peroxide toxicity in dental tooth whitening
have not been recognized and researchers have yet to definitively
determine the long term effects of hydrogen peroxide when used in tooth
bleaching agents.
Conclusion
What is evident from a review of the literature is the lack of consensus
in much of the research. Many areas of concern have not yet been
thoroughly investigated. It is well-documented that teeth can be bleached.
Most authors conclude that retreatment is necessary but disagree on the
intervals of time between treatments with reports ranging from one to
three years. Transient clinical side-effects such as thermal sensitivity
and mucosal irritation have been reported. Bleaching agents exert some
changes in hard and soft oral tissues and in restorative materials, although
it is uncertain if these changes are clinically significant. The short-term
effects on dental hard tissues and pulpal tissues appear to be reversible.
Questions about the frequent and/or long-term use of bleaching agents
and their impact on dental hard tissues, pulpal tissues and oral soft tissues
remain. Hydrogen peroxide agents pose some health risk concerns when
used in biological systems. The impact of hydrogen peroxide on human
oral mucosal antioxidant defense mechanisms is not yet completely
understood. Long-term scientific human studies are needed. Because
dental tooth whitening is likely to continue to be an available treatment
option, dental hygienists can use the current literature to educate the
public about the pros and cons of tooth whitening agents and procedures.
When bleaching procedures are to be implemented, dental hygienists can
ensure that the client is a non-smoker with healthy periodontium, has
no cervical erosion or enamel cracks, and has intact restoration margins.
Clients should be provided with custom-fitted bleaching trays with viscous
bleaching gel and be advised to follow instructions very carefully. Clients
should be firmly reminded not to retain the trays with bleaching agent
in their mouths overnight while sleeping, nor to increase the amount of
bleaching agent or the frequency of their use of bleaching agents without
first consulting a dental professional.