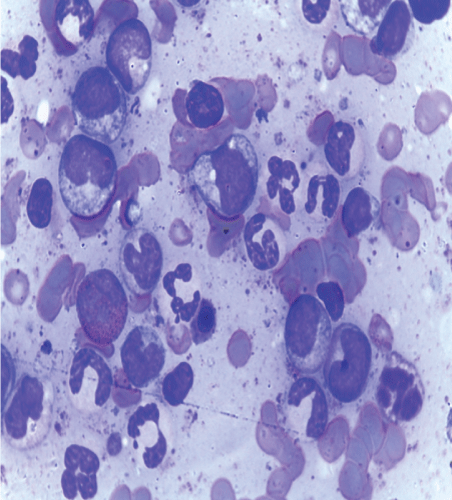
Figure 1: Bone marrow aspiration.

Samwel Jacob Rweyemamur* Joel Johanson James Pius Mwita Magesa Amar M Sawli Julieth Antony Batanyita Festo Kasmir Shayo Saning’o Sanget Cosolatha Mbatina Fredrick Jovin G Ngemela Atugonza Festo Shayo
Muhimbili University of health and allied sciences (MUHAS), department of Internal Medicine, Dar es salaam Tanzania*Corresponding author: Samwel Jacob Rweyemamu, department of Internal Medicine, Muhimbili University of health and allied sciences, Tanzania, Tel: +255787941606; E-mail: rsjoshua1972@gmail.com
A 25 Years old Woman with HIV stage four and Chronic Myeloid Leukemia. This patient was admitted to our hospital because of intra-abdominal mass for four months. The mass was insidious onset in which progressed gradually to increase in size towards the umbilicus causing early satiety. However the patient had a history of headache, dizziness and altered mental status. Also she presented a history of symptoms of severe anemia, B symptoms, bone pain and nose bleeding. There were no symptoms that could suggest GI or urinary system blood loss. She had no history smoking, exposure to chemotherapy, irradiation or repeated malaria infection. The lines of investigations showed normocytic anemia, Philadelphia chromosomes, and low CD4 count and high HIV viral load. She continued with anti-retrovirus treatment and received blood transfusion in our ward and was referred to Ocean Road Cancer Institute (ORCI) to start treatment with tyrosine kinase inhibitors.
Dual HIV infection and chronic myeloid leukemia exists in our patients. CML may be thought as a rare AIDs defined hematological malignancy in a WHO clinical stage 4.
For the best outcome of the patient, specific investigations and dual treatment for both HIV and CML is highly recommended.
The patient was apparently well until four months ago before admission when she noted an abdominal mass on the left upper part. The mass progressed gradually to increase in size towards the umbilicus causing early satiety. It was not accompanied by heart burn, difficult in swallowing, nausea, vomiting blood, passing blood in stool, diarrhea or constipation. There were no yellowish discoloration eyes, easy bruising, and swelling of the legs or joints. She had a normal micturition habit. However the patient had a history of headache, dizziness and altered mental status. Also there was a history of awareness of heart beat, difficulty in breathing on lying flat, cough, general body weakness and nose bleeding. She also had chest bone pain, low grade fever, profuse sweating and markedly weight loss. She had no known past history of schistosomiasis infection. She had no history of smoking cigarettes, treatment with radiation or chemotherapy therapy, pesticide contact, repeated episodes of malaria and a negative history of alcoholic use.
At a regional hospital, three month s ago she was diagnosed to have HIV disease and anemia with hemoglobin of 4 g/dl in which she started the anti-retroviral therapy Tenoforvir, Lamivudine, Efavirenz and received two units of blood. Her CD4 and viral load were not checked but the total white blood cell count showed leukocytosis and the provisional diagnosis of chronic myeloid Leukemia was made and were referred for confirmation and further management.
The patient dietary history was poor in terms of quality and quantity. However she had a normal gynecological history and there is no known family history of sickle cell disease or hematological malignancies in the family. In our department several investigations was done as presented on the table 1-3 and treatment commenced with hydroxyureas that caused abrupt pancytopenia and was stopped. She was monitored for pancytopenia and while on supportive management in our ward from December 2016 to January 2017 (2 months) and when the hemoglobin increase to 7gl/dl was sent to the Ocean Road Cancer institute (ORCI) for tyrosine kinase inhibitors. On follow up this patient for 14 days, she was showing clinical improvement while on treatment.
| Parameter | 10/12/2016 | 25/12/2016 | 16/1/17 PS*+CBC** |
24/1/17 PS+CBC |
Ref Range % |
| ESR mm/1hr | 150 | ||||
| WBC K/uL | 406 | 374 | 2.91 | 2.26 | 4 - 11 |
| Neutrophils asbs & (%) | 354(89.9%) | 167 (89.1%) | 1.39(47.8%) | 1.17(51.9%) | 37 - 80 |
| Lymphocytes abs & (%) | 5.86(24.9%) | 10.2(5.45%) | 1.16(49.7%) | 0.875(48.8) | 10 - 50 |
| Monocytes abs & (%) | 18.92(2.85%) | 8.07(4.31%) | 0.15 (5.16%) | 0.139(6.18%) | 0 - 12 |
| Eosinophils abs & (%) | 7, 21(1.27%) | 7, 21(1.27%) | 0.065(2.24%) | 0,2%(0.887) | 0 - 7 |
| Basophils abs & (%) | 0.946(0.161) | 0.836(0.46%) | 0.147(5.05) | 0.052 (0.052%) | 0 - 2.5 |
Table 1: Serial hematological results.
Note:
*Peripheral blood smear
**Complete blood count
| Parameter | 10/12/16 | 25/12/16 | 16/1/17 | 24/1/17 | Reference range |
| RBC | 1.566 | 3.06 | 2.42 | 2.1 | 3.88 - 5.46 |
| Hb g/dL | 4.68 | 8.48 | 6.38 | 5.81 | 12.1 - 15.9 |
| HCT | 13.94 | 14 | 2.6 | 17.9 | 34.3 - 46.6 |
| MCV fl | 87.6 | 91 | 85.2 | 85.2 | 80 - 97 |
| MCH pg | 59.8 | 27.6 | 26.4 | 27.6 | 27 - 31.2 |
| MCHC g/dL | 67.2 | 30.4 | 31 | 32.4 | 31.8 - 35.4 |
| RDW % | 0.93 | 26.1 | 24.5 | 22.4 | 11.6 - 14.8 |
| PLT | 186.6 | 121 | 77.6 | 60.1 | 150 - 450 |
Table 2: cont
| Parameter | 10/12/16 | 25/12/16 | 16/1/17 | 24/1/17 | Reference range |
| RBC | 1.566 | 3.06 | 2.42 | 2.1 | 3.88 - 5.46 |
| Hb g/dL | 4.68 | 8.48 | 6.38 | 5.81 | 12.1 - 15.9 |
| HCT | 13.94 | 14 | 2.6 | 17.9 | 34.3 - 46.6 |
| MCV fl | 87.6 | 91 | 85.2 | 85.2 | 80 - 97 |
| MCH pg | 59.8 | 27.6 | 26.4 | 27.6 | 27 - 31.2 |
| MCHC g/dL | 67.2 | 30.4 | 31 | 32.4 | 31.8 - 35.4 |
| RDW % | 0.93 | 26.1 | 24.5 | 22.4 | 11.6 - 14.8 |
| PLT | 186.6 | 121 | 77.6 | 60.1 | 150 - 450 |
Table 3: Serum chemistry.
On the physical examination, she is a wasted middle aged, fully conscious, not tachypnea and afebrile with 37 ºC temperature, pallor with no jaundiced. The patient had no signs that could suggest the presence of iron deficiency anemia or chronic liver disease. The vital signs were normal in which the pulse rate was 88 beats per minutes, blood pressure 120/75mmHg, respiratory rate 16 breath per minute, SPO2 was 98% in a room air, and random blood glucose were 5.0mmol/l. However she had oral thrush and inguinal lymph node palpable 1-1.5cms desecrate, non-tender but rubbery, other group of nodes were spared. The neck veins were not distended and there was no lower limb edema. She had hepatosplenomegally and no signs of ascites. All other systems had significantly normal finding.
On the review of laboratory results, the complete blood count shown leukocytosis in which the total count was 406 × 109 k/ul which later on fallen down after the initiation of hydroxy urea. The predominant cells were granulocytes and the hemoglobin level was quite low ranging from 4.68g/dl to 8.48 with MCV 91fl indicting normocytic anemia. The serum chemistry indicated only mild hypoalbuminemia and other parameters where within the acceptable range. Cytogenetics study detected the Philadelphia chromosome and BCR-ABL1/ ABL1 ratio=43%. The CD4+ of the patient is 66cells/micl and the HIV viral load was 16304cp/ml. The abdominal ultra sound showed hepatosplenomegaly the results which were similar to the abdominal CT scan with addition of multiple para aortic lymph node enlargement. The bone marrow aspiration showed all myelocyte cell lines with predominance of granulocytes, with blast cell less than 8%, few erythroids cells and Band forms. (Figure 1-3).

Figure 1: Bone marrow aspiration.
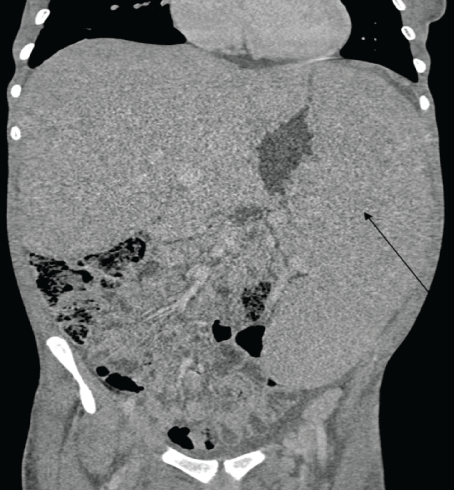
Figure 2: CT of abdomen showing hepatosplenomegally.
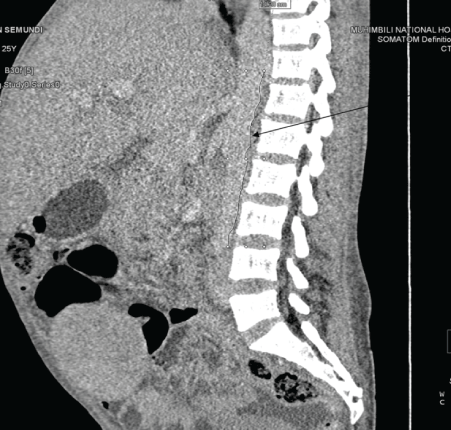
Figure 3: Showing multiple lymph nodes.
This patient is 25 years, immunocompromised with low CD4 count and high viral load despite of being on anti-retro viral for five months which was interrupted in the ward because she did not come with her medications. She is having anemia, B symptoms, oral thrush and hepatosplenomegally and multiple para-aortic lymph nodes on imaging. The liver function showed low albumin and on the cytogenetic study the Philadelphia chromosome was detected.
The patient came in our hospital with a confirmed diagnosis of HIV disease, the CTC card was reviewed which showed that the patient was on anti-retro viral therapy; tenofovir, lamivudine and efavirenz for five months. However she skipped the doses in our ward since she did not carry her Care and Treatment Card (CTC). HIV is a known cause of anemia, lymphadenopathy and in WHO clinical stage four the patients present with wasting syndrome, encephalopathy and oro-esophageal candidiasis [1]. This patient had clinical features that could put her in the WHO clinical stage four
This is the commonest AIDS defined hematological malignancy. This patient being HIV positive had 60 to 100 relative risk of NHL [2]. Also she had B symptoms (fever, weight loss, profuse night sweats) which could explain the clinical suspicious of lymphoma. These symptoms are presented in 30% to 40% of all patients with lymphoma [3,4]. Anemia, hepatomegaly is common and splenomegally is seen in 40% of patients with lymphoma. Hepatosplenomegally can be also a feature of T-cell lymphoma which cannot be excluded in this patient [5]. Lymphadenopathy and bleeding tendencies are also futures among patients with lymphoma [6]. However, this patient had approximately 1cm inguinal lymph node palpable with absence of cervical lymphadenopathy which is always present in 60% to 70% of patients with lymphoma h [3]. The lymph node was too small for a biopsy for histopathology diagnosis of NHL.
The patient presented with features of CML for the period of four months. She had splenomegaly which is accounting for about 48% to 76% of patients with CML [7] and hepatomegaly. Anemia although is a nonspecific sign, is encountered in 45% to 65% of patients with CML [8]. Our patient during the course of illness had severe malaria with Hemoglobin of 4g/ dl which required four units of blood transfusion. Bleeding tendencies, bone pain, fatigue, markedly weight loss, excessive sweating, low grade fever, reduced food intake, early satiety is pathognomonic features of CML [9]. Complete blood count showed leukocytosis with predominance of granulocytes and less 8% blasts which are consistence of CML in chronic phase. The cytogenetic confirmed the presence of Philadelphia chromosome (BCR-ABL1/ABL 43%) which is the all mark for CML [10].
HIV disease stage 4 chronic myeloid leukemia
HIV Disease and Chronic Myeloid Leukemia Is There Any Association
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasia associated with the deregulated proliferation of the myeloid lineage. It is cytogenetically characterized by the presence of the Philadelphia chromosome in 90% to 95% of patients, t (9;22) (q34;q11), which gives rise to the BCR-ABL1 fusion gene [11] that encodes a constitutively active tyrosine kinase responsible for the development of CML [12-14]. CML is a triphasic disease; Indolent or chronic phase (Blast cells <10%), accelerated phase (blast cells 10-20%) and blastic phase (blast cells >20%). Our patient was on chronic phase with blast cell of 8% [15].
CML accounts about 15% of all cases of leukemia and has a slight male predominance, M: F=1.6:1. The Median age at diagnosis 55-65 years and occurs in patients less than 20 years of age for 3% of cases. In Africa, the current incidence and prevalence data are missing and the available data do not distinguish CML from other types of leukemia. A small study in South Africa (2001) showed a crude incidence of leukemia of 2.08/100 000 for males and 1.44/100000 for females, with 458 and 338 new cases reported in that year, for males, and females respectively. Median age was 42 yrs. [8,16]. The findings reported from other African countries., showed the median ages of 38, 35, and 39 among newly diagnosed CML patients in Nigeria, Kenya and Malawi, respectively [17,18].
Patients who are infected with the HIV are at increased risk of developing malignancies. The AIDS defining malignancies which are well documented constitutes the Kaposi’s sarcoma, NHL and invasive carcinoma of the cervix, CML has rarely been described in association with HIV infection [2,16]. The HIV patients with CML which are reported are less than 50, and more than half of these are from the sub-Saharan Africa [2].
HIV as well as AZT (azidothydimine/zidovudine) an antiretroviral agent is known to cause myelodysplasia. However, there are no reports of the myelodysplasia evolving to CML. Our patient at a time of provisional diagnosis CML was ARV naïve, which makes the negative connection with her treatment for HIV.
It is therefore plausible to assume that the association between CML and HIV is likely to be coincidental rather than causal [19].
In the case series of 18 patients with HIV and CML which was reported in South Africa Soweto and Johannesburg between January 1991 and June 2011 by Moosa Patel, 72% of all patients were newly diagnosed of both diseases simultaneously. Based on a high risk Sokal score, 89% of these patients presented with advanced CML compared to their seronegative and 44% of patients had an accelerated and blast phase disease [19-21]. From this observation, it can be presumed that HIV fuels CML advancement.
The combination of both Tyrosine kinase inhibitors and HAART provides tremendous improvement in terms of survival and mortality. Six patients with a diagnosis of HIV and CML were treated with TKI’s (tyrosine kinase inhibitors) and antiretroviral therapy (ART) irrespective of their CD4 count had a more favorable outcome than the 12 patients who did not have access to the TKI’s, in particular Imatinib [19]. Another single center report from South Africa shown a successfully management of 10 HIV positive with low CD4 and CML [22]. Our patient is currently on both HAART and TKIs at ORCI.
Dual HIV infection and chronic myeloid leukemia exists in our patients. CML may be thought as a rare AIDS defined hematological malignancy in a WHO clinical stage 4.
For the best outcome of the patient, specific investigations and dual treatment for both HIV and CML is highly recommended.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Rweyemamu SJ, James JJ, Magesa PM, Sawli AM, Batanyita JA, et al. (2018) A Case Report of Muhimbili National Hospital Case 1-2017: A 25 Years Old Woman with HIV and AIDS and Chronic Myeloid Leukemia. Clin Res Open Access 4(3): dx.doi.org/10.16966/2469-6714.134
Copyright: © 2018 Rweyemamu SJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access