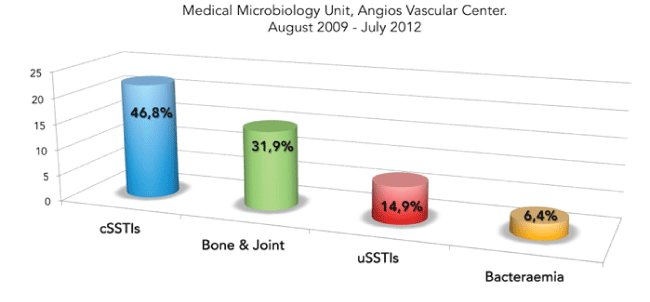
Figure 1: Infections most frequently treated using Daptomycin.

Marcel Marcano-Lozada1,2* Silvia Molero-Leon3,4
1Medical Microbiology Unit, Angios Vascular Center and Wound Clinic, Caracas, Venezuela*Corresponding author: Marcel Marcano-Lozada, Medical Microbiology Specialist, Medical Microbiology Unit, Angios Vascular Center and Wound Clinic, Caracas, Venezuela, Tel: +506 57190516; E-mail: marcelmarcano@gmail.com
Objectives: To describe the Venezuelan clinical and microbiological 3 years experience with the use of daptomycin in a reference vascular center and wound clinic included in the EUCORE(SM) (European Cubicin® Outcomes Registry and Experience) study, as well as the efficacy and safety outcomes.
Materials and methods: EUCORE(SM) is a European multicenter continuous record extending to Latin America, retrospective, non-comparative postmarketing (Phase IV) in which patients who have been administered at least 1 dose of DPC are included; whose primary objective is to evaluate the clinical outcome. Patients enrolled in one of the two Venezuelans participating centers among August 2009 and July 2012 (RP3, RP4, and RP5) were analyzed.
Results: 47 patients (27 males) were included; aged between 16 and 89 years (average 60 years), age over 65-55.4% (26); and presenting comorbidities 41 patients (87.2%), the most common was a peripheral vascular disease. Daptomycin was used as Outpatient Parenteral Antimicrobial Therapy (OPAT), at doses of 4, 6 or >6mg/kg/day on 31, 8 and 8 (66, 17 and 17%) patients respectively; with mean treatment duration of 15.3 days (1-42 days). The main treated infections were complicated skin and soft tissue (22-46.8%), musculoskeletal (15-31.9%), uncomplicated skin and soft tissue (7-14.9%) and endovascular prosthesis associated-bacteremia (3-6.4%). The most frequently treated isolated was Staphylococcus aureus (39-83% and 95% resistant to methicillin), coagulase-negative staphylococci (2-4.3%) and others (12.7%). Daptomycin resistance was absent (MICs over 1 μgr/mL) and a reverse Creep MIC was observed between 2009-2012, contrary to what was observed with vancomycin (MICs ≤ 0.094 to 2 μgr/mL). The overall treatment success rate was 77% (healing), it was used in 81% of patients as a rescue medication (second or third therapeutic option). 6 adverse events were documented in the first reporting period -RP3- (severe fatigue), which not forced discontinuation of the drug in any patient (further analysis showed no causality in any case), and no reports of nephrotoxicity, pneumo or muscle toxicity were observed.
Conclusions: Daptomycin has an excellent efficacy and safety profile, has ideal characteristics for use in OPAT, even for prolonged periods, in elderly patients with multiple comorbidities.
Daptomycin; EUCORE; Gram-positive; Venezuela; Safety; Efficacy
In Venezuela, Gram-positive severe infections present a challenge for the physicians, because every health care center follows treatment protocols according to its own microbiology isolates susceptibility aligned with national or international therapeutic guidelines. In addition, elderly or immunosuppressed patients have a lot of comorbidities, that difficult the selection of the right antimicrobial therapy.
Daptomycin (Cubicin®), the first-in-class cyclic lipopeptide antibiotic, was approved in Europe for the treatment of Complicated Skin and Soft Tissue Infections (cSSTIs) in 2006 and for the treatment of right-sided infective endocarditis (RIE) due to Staphylococcus aureus and S. aureus bacteraemia (SAB) when associated with RIE or with cSSTI in 2007 [1]. It was available in Venezuela since 2009 and represents an opportunity to improve severe Gram-positive infections treatment.
Daptomycin is bactericidal against Gram-positive bacteria, including Methicillin-Resistant Staphylococcus aureus (MRSA) and Vancomycin-Resistant Enterococci (VRE), it kills Gram-positive bacteria by a novel mechanism of disruption of multiple bacterial plasma membrane functions, without penetrating the cytoplasm [2]. Insertion of the lipophilic daptomycin tail into the bacterial cell membrane with oligomerization and channel formation causes rapid membrane depolarization and a potassium ion efflux. The arrest of DNA, RNA, toxin production, and protein synthesis follows, resulting in bacterial cell death without lysis of the cell wall, which gives a further advantage in diseases where inflammatory response associated counter antimicrobial use producing cell lysis [3-5].
The in vitro potency of daptomycin has been demonstrated against a wide range of aerobic and anaerobic Gram-positive bacteria, including MRSA, glycopeptides-intermediate S. aureus (GISA) vancomycinresistant S. aureus (VRSA), methicillin-resistant coagulase-negative staphylococci (MRCNS), and VRE. Synergy with daptomycin has been described in vitro with aminoglycosides, i.e., gentamicin, oxacillin, other b-lactams, macrolides, and rifampicin. Daptomycin exhibits a dose-dependent post-antibiotic effect lasting from 1 to 6 hours against E. faecalis and S. aureus after exposure to concentrations ranging from 0.25 to 16 mg/L (i.e., between one- and eightfold the MIC) [2,6-8].
Pharmacology of daptomycin is interesting, with an 8-hours halflife, once-daily dosing results in linear pharmacokinetics at doses up to 12 mg/kg, with minimal drug accumulation. Daptomycin distributes primarily in the plasma, with penetration to vascularized tissues. The drug is highly protein-bound (92%); excretion occurs primarily via the kidneys. Approximately 80% of the total dose, of which two-thirds is an intact drug, is recovered in the urine. In patients with severe renal impairment (creatinine clearance <30 mL/min), the dosing interval is increased from once daily to every 48 hours. Daptomycin’s unique mechanism of action and its lack of metabolization by cytochrome p450 or other hepatic enzymes results in an absence of drugdrug interactions. Synergistic interactions were observed between daptomycin and gentamicin against staphylococci and enterococci, including strains resistant to methicillin and vancomycin (valuable to biofilm disease infections treatment); in vitro, synergistic interactions of daptomycin and rifampin have been shown against MRSA, VRSA, and VRE, with a great antibiofilm activity and reduction of the rifampicin resistance appearance. Not antagonism interactions were observed with daptomycin use in combination with several antimicrobial agents, only additive, synergistic effect or indifference was reported.
Skeletal muscle was the most sensitive tissue on the adverse effects of daptomycin in animal studies. Mild myopathy was easily predicted and monitored by measuring serum creatine phosphokinase concentrations; the effect was reversible upon the cessation of therapy [9]. As a national reference center for patients with chronic wounds in limbs, especially legs, Angios Vascular Center and Wound Clinic specializes in saving limbs at risk of amputation for various reasons such as peripheral vascular disease (arterial and/or venous) diabetes mellitus, trauma, severe infections, etc.
A multidisciplinary approach form different medical and surgical specialties is the key to heal the chronic wounds associated to osteomyelitis, specially diabetic patient with peripheral vascular diseases (venous, arterial or mixed), and becomes a therapeutic challenge, due to alterations in healing process for metabolic compromise or tissue oxygenation, immunocompromised situation secondary to diabetes, antimicrobial use restrictions due to advanced age, renal and hepatic impairment, allergies; multidrug-resistant pathogens (because many patients come from healthcare centers or nursing houses).
In addition, the actual way to precognize ambulatory therapy (even parenteral) is key in our center, because it comes in parallel with costs reduction, low healthcare-associated infections rate, quickly recovery and life quality improvement. We prefer the use of outpatient parenteral antimicrobial therapy (OPAT) for the management of cSSTI, osteomyelitis and even septic arthritis based on the previous reasons.
Clinical experience with daptomycin in Europe since its approval has been captured by the EUCORE(SM) (European Cubicin® Outcomes Registry and Experience) study, a multicentre, retrospective, non interventional registry sponsored by Novartis Pharma AG that collected data across 18 countries (Europe, Latin America and Asia). EU CORE(SM) was designed to collect data on the characteristics patient population, infections, pathogens and adverse events and clinical outcomes of patients receiving at least one dose of daptomycin. However, because of their rigorous nature (e.g. inclusion and exclusion criteria and the requirement for protocol adherence), clinical trials may not always reflect the true experience with a drug in clinical practice, patient registries such as EUCORE(SM) provide insight into real-world clinical experience with daptomycin and build on the evidence base from clinical trials and “customized” for every country. This retrospective report describes the data from 3 years of daptomycin use at the real-world setting for the treatment of Gram-positive infections in Venezuela, from August 2009 to July 2012, as part of 3 periods of EUCORE(SM)) study data collection, for patients who had received at least one dose of daptomycin, with a two-year follow-up data collection until 2014 for patients with endocarditis, intracardiac/ intravascular device infection, osteomyelitis, or orthopedic device infection (completely data not published) [10,11].
EUCORE(SM) is a European multicenter continuous record extending to Latin America, retrospective, non-comparative postmarketing in which patients who have been received at least 1 dose of daptomycin are included; whose primary objective is to evaluate the clinical outcome. Patients enrolled at Angios Vascular Center and Wound Clinic in one of the two Venezuelans participating centers between August 2009 and July 2012 (RP3, RP4, and RP5) were analyzed.
Patients included were diagnosed with uSSTIs (uncomplicated skin and soft tissue infections), cSSTIs, osteomyelitis, endovascular prothesis-associated infections, septic arthritis and diabetic foot severe infections. Data were collected from Clinical Report Forms (CRFs), and reflected the most important findings from clinical records, then spreadsheets were composed to organize information and promote the analysis using comparative statistics (percentages) and summarized.
A total of 47 patients (27 males/20 females) were included; aged between 16 and 89 years old (average 60 years old), age over 65 years in 26 (55.4%) patients. This high number of elderly patients supposed an increased immunosuppression condition age-related, with an important number of it with comorbidities 41 of 47 patients (87.2%), the most common was peripheral vascular disease (PVD) (16-39%), diabetes mellitus (DM) (11-26,9%), PVD+DM (6-14,6%), fractures (3-7,3%), oncological malignancies (3-7,3%) and renal transplant (2- 4,9%), 6 patients no present comorbidities.
In the case of PVD, peripheral obstructive arteriopathy (POA) overlap peripheral venous disease (PVD) with 18 cases versus 2, and 4 patients with mixed pathology (POA+PVD). This vascular deficiency conditioning antibiotic distribution on infected tissues and healing capacity related to critical ischemia and dry gangrene.
Type 2-diabetes predominates with 8 patients over 3 with Type 1 disease, it could be explain because this patient goes to healthcare service later than type 1 where a metabolic decompensation (diabetic ketoacidosis or hyperosmolar coma) comes associated with the infectious process, but Type 2 patients not necessarily present this severe metabolic decompensation related to the infection. The combination of PVD+DM had the worst initial prognosis, but finally a satisfactory therapeutic response [12].
3 patients with fractures associated with osteomyelitis, had an excellent therapeutic response, an in all 3 cases, dual antimicrobial combination therapy was a standard selection.
The main treated infections were complicated skin and soft tissue (22-46.8%) with severe diabetic foot infection (DFI) in 11 patients; musculoskeletal infections included osteomyelitis [12] and arthritis [3] (15-31.9%), uncomplicated skin and soft tissue (cellulitis, minor abscess) (7-14.9%) and endovascular prosthesis associated-bacteremia (3-6.4%) (Figure 1).

Figure 1: Infections most frequently treated using Daptomycin.
The most frequently isolated treated was Staphylococcus aureus (39- 83%), and 95% of it were resistant to methicillin (MRSA), and more than a half of these were biofilm producers-25 isolates); coagulasenegative staphylococci (2-4.3%) and others (12.7%). MRSA strains lacking yellow-golden pigment production predominated (37 of 39), these being associated with severe subacute or chronic and deep tissue infections, instead of surface infections caused by pigmented strains (e.g. uncomplicated cellulitis), being this feature phenotypic importance to the treating physicians because as evidenced in this study, are associated with increased antimicrobial resistance and biofilm production (Figure 2).
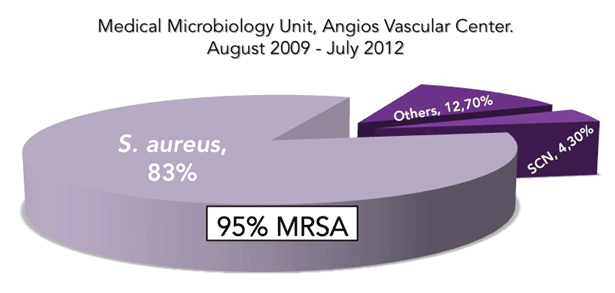
Figure 2: Microorganisms most frequently treated using Daptomycin.
Daptomycin was used as Outpatient Parenteral Antimicrobial Therapy (OPAT) [13-15], in all the patients, they arrived at the medical center, to an special ambulatory treatment facility room, and received the daptomycin infusion for a 30 minutes time or the bolus injection in 2-5 minutes time [16], at doses of the approved doses of 4 and 6mg/ Kg/day or >6mg/kg/day (unapproved dose, used based on anti-biofilm previous successful reported experiences) on 31, 8 and 8 (66, 17 and 17%) patients respectively; with mean treatment duration of 15.3 days (1-42 days), for uCSSTI, a mean of 4 to 7 days was the most frequent, but long treatment regimens were necessary to severe disease as chronic osteomyelitis bacterial eradication [17] (Figure 3).
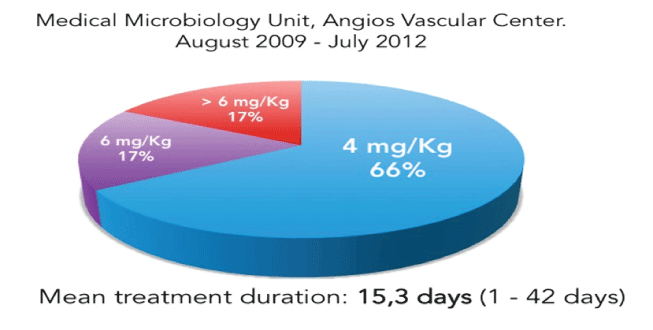
Figure 3: Daptomycin doses in 47 patients.
The overall treatment success rate was 77% (complete healing), it means that 36 patients achieve bacterial eradication (13 osteomyelitis, 11 with DFI, 3 with uCSSTI, 3 with septic arthritis and 3 with bacteremia related to endovascular prosthesis). This demonstrated the high power of Daptomycin in severe infections, and its value in biofilm-related disease.
It was used in 81% (38) of patients as a rescue medication (second or third therapeutic option, combined with macrolides or rifampicin for biofilm producer bacteria or is biofilm disease is highly suspicious).
Maybe if daptomycin had been considered as the first-line drug for these patients, the treatment success rate would be higher in this experience; however, the success of unwieldy pathologies as chronic osteomyelitis, endovascular prosthesis associated infections, septic arthritis and DFI, leave no doubt of its place within the vademecum for management of severe infections caused by resistant Gram-positive bacteria [12,18].
Daptomycin resistance was absent, with Minimal Inhibitory Concentrations (MICs) below the cut-off of 1 μgr/mL, the absence of resistance could be explained because Daptomycin is a brand new antimicrobial agent, and bacterial isolates never facing and lack crossover resistance mechanism due to its unique mechanism of action in the bacterial plasmatic layer. So, a MIC Creep phenomenon was not observed in this 3 year period (there was no increase in MIC values within the range of susceptibility).
A “Reverse MIC Creep phenomenon” (reduction of the MIC values inside the “susceptible” range) was observed between 2009-2012, when initial MICs values were over 0,047 μgr/mL at the first report period (2009), and reduces to a mean of 0,023 μgr/mL for the last reported period (2012). A plausible explanation for this could be the high cidality (bactericidal) power of the drug, and the growth in its use.
A “MIC Creep phenomenon” (growing MIC values inside “susceptible” range), was observed for vancomycin (MICs ≤ 0.094 to 2 μgr/mL), and could be explained for the increase spread of isolates with an expanded murein wall formation, that leads to the “Vancomycin trap” or “Vancomycin jail”, when the antimicrobial molecules were “trap” by an enhanced number of therapeutic targets, without an opportunity to inhibit the bacterial wall growth [18-23].
The pharmacovigilance reveals that 6 adverse events (AE) were documented in the first reporting period at the year 2009-RP3- (severe fatigue), which not forced discontinuation of the drug in any patient (further analysis showed no causality in any case). The management of this AE leads to a closer daily monitoring of creatine phosphokinase (CPK) values, increase parenteral hydration, but not leads to a dose reduction, because the patients were strictly followed in order to maintain the bacterial eradication benefit of the antimicrobial agent use. All the patients who received statins they should discontinue use during antimicrobial therapy, to reduce the risk of muscle toxicity. No reports of nephrotoxicity, pneumo or muscle-toxicity were observed in these 3 years experience, similar to another investigators findings [18,24,25].
This real world 3 years experience shows that Daptomycin has an excellent efficacy and safety profile, especially for severe infections related to biofilm disease and caused by multidrug-resistant microorganism, has ideal characteristics for use in OPAT, even for extended periods, and in elderly patients with multiple comorbidities, and an excellent safety profile due to few and mild adverse events. These results were similar to the reported by other groups across the world [10,11,18,24,25].
Download Provisional PDF Here
Article Type: Research Article
Citation: Marcano-Lozada M, Molero-Leon S (2018) 3 Years of Clinical and Microbiological Experience with Daptomycin in a Vascular Center and Wound Clinic in Caracas, Venezuela. J Clin Lab Med 3(1): dx.doi.org/10.16966/2578-9578.120
Copyright: © 2018 Marcano-Lozada M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access