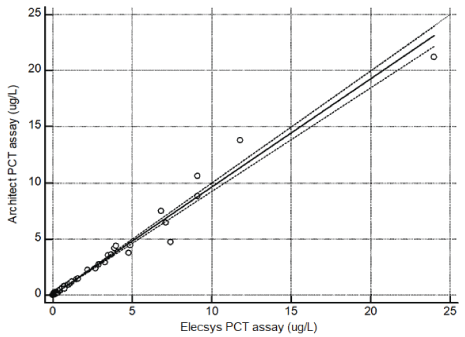
Figure 1: Linear regression of Architect PCT and Elecsys PCT

Eddie Chi Man LEUNG* Ching In HAU Raymond Wai Man LAI
Department of Microbiology, Prince of Wales Hospital, Hong Kong, China*Corresponding author: Eddie Chi Man Leung, Department of Microbiology, Prince of Wales Hospital, Hong Kong, China, Tel: + 852-3505 3342; E-mail: lcm414@ha.org.hk
Objectives: To compare the new Abbott Architect PCT immunoassay with the established of Biomerieux Vidas and Roche Elecsys PCT methods.
Methods: Total of 78 frozen samples previously tested on Elecsys PCT assay were analysed by Architect and Vidas methods and compared using regression analysis, Bland-Altman difference plot, and analysis of concordance at three clinically diagnostic PCT level cut-offs at 0.1, 0.25 and 0.5 ug/L.
Results: Architect PCT assay correlated well with Vidas (R=0.954) and Elecsys(R=0.972) assays. The diagnostic agreement with the two reference methods at three clinical relevant cut-offs was excellent with 93.6-94.8% at 0.1 ug/L, 91.0-93.6% at 0.25 ug/L and 98.7-100% at 0.5 ug/L.
Conclusion: The results of our investigation demonstrated that the new Architect BRAHMS PCT assay correlated well with the Vidas and Elecsys PCT methods compared. The Architect PCT assay is suitable for clinical diagnostic use for PCT measurement.
Procalcitonin; Architect PCT; Immunoassay; Sepsis
Procalcitonin (PCT), the precursor of calcitonin, is a biomarker used for the diagnosis of bacterial infection and sepsis. It has been well established that levels of PCT significantly increase upon infections and decrease upon recovery. Clinical PCT level measurement has gained worldwide acceptance in the last 10 years [1]. PCT is a functional protein consisting of 114 to 116 amino acids. It is an immune modulator with various functions, differentiating it from other groups of previously known inflammatorily active molecules, such as cytokines, leukocytary surface markers, acute phase proteins, or clinically detectable signs of inflammation. PCT has special functions and the body regulates its level very strictly, and it is a particular good indicator of the presence of a bacterially induced systemic inflammatory reaction [2].
Bacterial infections characterized by a systemic inflammatory reaction induce PCT, resulting in elevated its levels. Serum or plasma concentration of soluble PCT levels in healthy people is generally below 0.1 ug/L, whereas for levels over 0.5 ug/L, sepsis or a systemic inflammatory reaction should always be considered [2,3].
After the development of first BRAHMS PCT Kryptor method which has developed from the original BRAHMS luminometric immunoassay (LIA) [4], several diagnostic companies, such as Biomerieux and Roche, have partnered with BRAHMS for development automated PCT assays on their propriety platforms by different technologies [5,6]. Recently, Abbott has developed a new automated quantitative BRAHMS PCT assay run in Architect platform. In this study, we compared the analytical performance of this newly developed PCT assay with the Biomerieux Vidas and Roche Elecsys PCT assays in serum samples.
From February to July 2017, a total of 78 specimens for routine PCT measurement as received by Department of Chemical Pathology, Prince of Wales Hospital, Hong Kong were first analysed using the Elecsys PCT immunoassay. After routine testing, the aliquots of serum specimen were stored at-20oC until further analysis.
For comparison study, the aliquots were then thawed at room temperature, mixed thoroughly and then analysed by Vidas and Architect for PCT measurement on the same day. The analytical performance of Architect PCT assay was compared with results obtained from the Vidas and Elecsys PCT assays.
The Architect PCT assay is a two-step immunoassay for the quantitative determination of PCT in serum and plasma using chemiluminescent micro particle immunoassay (CMIA) technology on the automated Architect System analyser. The PCT present in the sample binds to the anti-PCT coated micro particles. After washing, anti-PCT acridinium labelled conjugate is added to create a reaction mixture. Following another wash cycle, pre-trigger and trigger solutions are added to the reaction mixture. The resulting chemiluminescent reaction is measured as relative light units (RLUs). There is a direct relationship between the amount of PCT in the sample and the RLUs detected by the Architect System optics. The calibration of the assay requires 6 calibrators in duplicate. The sample volume of the assay is 150 uL and the measuring interval is 0.02 to 100 ug/L with the highest observed Limit of Detection (LoD) value of 0.00 ug/L and Limit of Quantification (LoQ) of 0.01 ug/L. In this study, the Architect PCT assay was run on i1000 analyzer.
For the comparison PCT immunoassay, Vidas PCT assay was run on mini VIDAS analyser by enzyme-linked fluorescent assay (ELFA) and Elecsys PCT was run on Cobase601 analyze employed electrochemiluminescence immunoassay (ECLIA) for PCT assay determination. According to the manufacturers’ information, the detection limit (LoD) of Vidas and Elecsys PCT assays is 0.03 ug/L and ≤ 0.02 ug/L respectively and functional sensitivity (LoQ) of Vidas and Elecsys is 0.05 ug/L and 0.06 ug/L respectively.
All three assays were calibrated and quality control materials were analysed according to the manufacturer’s instruction. The results of the control materials were within the respective manufacturer’s limits.
Three levels controls was analysed for PCT determination in 5 replicates per run on 5 different days on Architect i1000 analyzer for the estimation of intra-assay and inter-assay imprecision. The study is following the Clinical and Laboratory Standards Institute (CLSI) guidelines EP15 A3 [7].
The analysis was carried out using linear regression analysis, Spearman’s correlation coefficient and Bland-Altman different plot to compare the different measurement methods [8] and agreement at three clinical diagnostic cut-offs at 0.1, 0.25 and 0.5 ug/L was calculated with kappa statistic [9]. The level of statistical significance was set at p<0.05. The statistical analyses were performed using MedCalc, version 15.0 (MedCalc Software, Ostend, Belgium).
A good correlation was observed when comparing Architect PCT assay with Elecsys and Vidas PCT assays for 78 specimens over a range of 0.05 to 24 ug/L. In details, the linear regression coefficient (R) between Architect with Elecsys was 0.972 (Figure 1, y=0.014+0.962x) and between Architect and Vidas was 0.954 (Figure 2, y=0.024+0.773x).

Figure 1: Linear regression of Architect PCT and Elecsys PCT
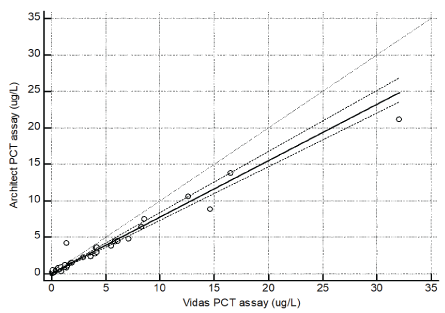
Figure 2: Linear regression of Architect PCT and Vidas PCT
The results of Bland-Altman plot analysis are shown in Figures 3 and 4. The mean bias between Architect and Elecsys was -0.1 ug/L with upper and lower 95% limits of agreement of -1.2 to 1.0 ug/L (Figure 3). The mean bias between Architect and Vidas was -0.5 ug/L with upper and lower 95% limits of agreement of -3.5 to 2.5ug/L (Figure 4).
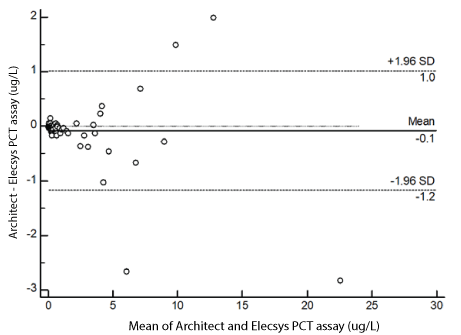
Figure 3: Absolute difference Bland-Altman plot for PCT (ug/L) between Architect PCT and Elecsys PCT. Solid line is the mean bias; dashed lines are upper and lower level of agreement at 95% confident interval
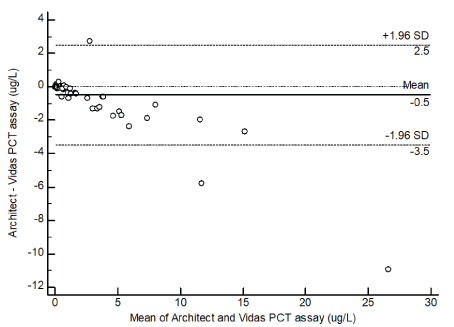
Figure 4: Absolute difference Bland-Altman plot for PCT (ug/L) between Architect PCT and Vidas PCT. Solid line is the mean bias; dashed lines are upper and lower level of agreement at 95% confident interval
The diagnostic performance of Architect PCT assay was assessed in comparison with Vidas and Elecsys PCT assays at the clinical relevant cutoffs of 0.1, 0.25 and 0.5ug/L (Table 1).The results suggest that Architect PCT assay exhibit comparable performance with two reference methods at the three relevant diagnostic thresholds of 0.1, 0.25 and 0.5 ug/L. In particular, an excellent agreement was found between Architect and Vidas, the diagnostic agreement between was 93.6% (κ, 0.84) at 0.1 ug/L, 93.6% (κ, 0.82) at 0.25 ug/L and 98.7% (κ, 0.97) at 0.5 ug/L thresholds. The agreement between Architect and Elecsys was excellent at 0.25 ug/L and 0.5 ug/L with 93.6% (κ, 0.87) and 100% (κ, 1) respectively.
| Procalcitonin cut- off (ug/L) | Elecsys | Vidas |
| 0.1 | 93.6%; κ, 0.76 (95% CI 0.56 -0.96) |
94.8%; κ, 0.84 (95% CI 0.68 -0.99) |
| 0.25 | 93.6%; κ, 0.87 (95% CI 0.766 -0.98) |
91.0%; κ, 0.82 (95% CI 0.69 -0.495) |
| 0.5 | 100.0%; κ, 1 (95% CI 1.00 -1.00) |
98.7%; κ, 0.97 (95% CI 0.92 -1.00) |
Table 1: Percentage of agreement and value of kappa obtained with Architect PCT assay compared to Elecsys and Vidas PCT assay at three different diagnostic thresholds
A summary of the results is listed in Table 2. The average intra-assay and inter-assay imprecision for the controls with three PCT concentration levels was 3.49% and 1.25% respectively
| levels | N | Intra-assay | Inter-assay | ||
| Mean | CV (%) | Mean | CV (%) | ||
| L | 25 | 0.20 | 3.16% | 0.20 | 1.13% |
| M | 25 | 1.96 | 1.68% | 1.96 | 0.90% |
| H | 25 | 68.41 | 5.62% | 68.41 | 1.73% |
| Average | 3.49% | Average | 1.25% | ||
Table 2: Precision data of Architect PCT assay
Procalcitonin can be useful test for diagnosis and prognosis of bacterial infection and it is usually ordered, along with other tests such as blood culture or sputum culture, to help diagnose sepsis or bacterial respiratory tract infection [10,11,2]. In addition, monitoring the PCT concentration would provide guidance to antibiotic treatment and may reduce in antibiotic usage without affecting the patient outcome. This is particular important for critically ill patients in intensive care unit.
In this study, the performance of the new automated Abbott Architect PCT assay was evaluated. The assay was compared with two reference methods, Vidas and Elecsys PCT assays. The measuring intervals of the three assays differed, with the Architect from 0.02 to 100 ug/L, Vidas from 0.05 to 200 ug/L and Elecsys from 0.02 to 100 ug/L. The overall bias of Architect versus Elecsys was -0.1 ug/L and Vidas was -0.5 ug/L. The distribution of the differences with Architect and Elecsys was not significant between 0.05 to 24 ug/L. The difference with Architect and Vidas was modest at PCT concentration below 10 ug/L whereas a negative bias was observed above 10 ug/L. However, this small difference does not substantially impact on the diagnosis of bacterial infections according to the current diagnostic cut-off.
The results of our comparison suggest that the different reagents and techniques exhibit comparable performance at different relevant diagnostic thresholds at 0.1, 0.25 and 0.5 ug/L. In particular, the agreement was excellent at the diagnostic cut-off at 0.5 ug/L which reflect moderate risk for progression to severe systemic infection. The patient in this category should be closely monitored both clinically and reassessing the PCT level within 6-24 hours, however, we have shown in this study that PCT level measured by different methods with significant bias was observed. Thus, PCT measurement should not be used interchangeably with other methods for monitoring patients.
For laboratory choosing methodology for PCT assay, the cost of the assay is one of the important factors to consider. The calibration of Architect PCT assay required to run six calibrators in duplicates in a 30-day recalibration frequency. This may have extra cost in reagents for calibration of the assay when the laboratory is running low volume of samples.
There are some limitations in this study, the analytical range (0.02 -100 ug/L) of Architect PCT assay was not fully evaluation, with samples only covered from 0.05 to 24 ug/L and mostly below 5 ug/L. We have shown good correlation of PCT below 25 ug/L in this study, however, patients with high procalcitonin (>100 ug/L)considered as independent predictors of poor prognosis including sepsis, trauma complicated with respiratory and circulatory insufficiency in intensive care unit has been reported [12]. Therefore, further evaluation of high procalcitonin level should be considered. In addition, due to the limited number of available reagents, the sensitivity, specificity and linearity of Architect PCT assay were not evaluated in this study.
The results of our investigation demonstrated that the new Abbott Architect PCT assay correlated well with the Vidas and Elecsys PCT methods compared. Nevertheless, the varying bias between different methods indicates that longitudinal patient monitoring should be preferably carried out using the same immunoassays. In conclusion, the Abbott Architect PCT assay is suitable for clinical routine diagnostics use for PCT measurement.
Download Provisional PDF Here
Article Type: Research Article
Citation: LEUNG ECM, HAU CI, LAI RWM. (2017) Comparison of a New Automated Abbott Architect Procalcitonin Assay with Biomerieux Vidas and Roche Elecsys Methods. J Clin Lab Med 2(2): doi http://dx.doi.org/10.16966/2572-9578.117
Copyright: © 2017 LEUNG ECM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access