Introduction
Aging of the skin can be attributed to continuous external insult from innate and external factors, resulting in increased wrinkling, sagging, laxity, and uneven skin texture [1]. Aged skin, especially photoaged skin, is coarsely wrinkled and manifests as a decrease in skin thickness and elasticity, dryness, distorted barrier function, and altered penetrability and pigmentation [2]. The process is characterized by deterioration of the skin and damage to collagen. Matrix Metalloproteinases (MMPs) appear to play a major role in mediating long-wave ultraviolet (UVA) radiation-induced skin aging [3-5].
Intrinsic aging is primarily caused by accumulated damage due to free radicals and reactive oxygen species-induced damage to critical macromolecules. As a result, the aged skin has a wrinkled appearance and a higher chance of developing skin disorders [6]. The processes of angiogenesis, lipid and sweat production, immune function, and Vitamin D synthesis are also delayed, resulting in a decrease in the ability to heal wounds, increased atrophy, greater vulnerability to external factors, and increased growth of benign and malignant skin diseases [7]. The cumulative effect leads to a reduction in the durability and physiological function of the human skin [8]. Understanding the critical process of skin aging is essential for establishing improved skin care products which can reduce the impact of age-related factors [9].
Apigenin is naturally occurring plant flavones common to many fruits and vegetables with a variety of properties beneficial to skin care emerging in the recent literature. Apigenin can scavenge free radicals as an antioxidant and contains anti-inflammatory and anti-carcinogenic properties, and it can restore skin damage from exposure to UVA and short-wave ultraviolet (UVB) radiation [2,3,10-12]. One study reported apigenin use could protect against and decrease the activity of MMP-1, an endopeptidase which destroys the collagen matrix [13]. A disrupted collagen matrix results in decreased elasticity and dryness. Choi S et al. [14] reported that an apigenin-based cream could increase dermal density, improve elasticity, reduce the length of fine wrinkles, improve tone evenness, moisture, and Transepidermal Water Loss (TEWL), and may be a promising anti-aging agent. Apigenin can inhibit the expression of Cyclooxygenase-2 (COX-2; a mediator of inflammation), an ability which is thought to be a factor in apigenin’s antitumor activity. Given that inflammation from UVA and UVB radiation damage further contributes to skin aging, this anti-inflammatory aspect further aligns with apigenin’s anti-aging properties [10,11]. Britto SM et al. [15] reported that apigenin could protect skin cells against UVBinduced cyclobutane pyrimidine dimer formation. Given these reported anti-aging and anti-photoaging effects, we explored the use of an apigenin-based face cream to assess its effect on aging in human skin. Based on previous articles regarding the ability of apigenin to decrease the appearance and effects of photoaging in vitro and in vivo, we expected to see a clinical demonstration of improvement in skin appearance. Objectively and subjectively, the expected results were an improvement in skin texture, improved patient perception of youthfulness, and some improvement in elasticity and firmness.
The goal of the study was to evaluate apigenin’s efficacy in reducing the appearance of aging and to assess previous in vitro and in vivo reports that apigenin-based creams can improve wrinkling, skin elasticity, firmness, evenness, brightness, dullness, and other features often associated with the photoaging process. The overall goal was to see if an apigenin-based cream can influence the effects of photoaging.
Materials and Methods
This study was conducted in accordance with an ethics committee from Evaulab. The standard procedure and associated documents were reviewed and approved prior to the beginning of the study by an Ethics Committee (an independent organization whose responsibility is to ensure the protection of the rights, safety and well-being of the participants participating in the study). The data obtained for each volunteer were recorded on individual case report forms. The study was an open-label centered design, meaning the investigator, participants, and sponsors were aware of the nature of the test materials. The study consisted of 8 weeks (56 days) of use by the participants of a 10% apigenin-containing skin care regiment, and an assessment was conducted on cutaneous aging in a sample population of women over 30 years old. Data for the study were collected on day 0 (for baseline), day 28 (4 weeks; the midpoint of the study), and on day 56 (8 weeks; the final day of the study).
A total of 25 healthy female volunteers over age 30 were included in the study. Inclusion criteria included female subjects in good health, above age 30 with fine lines and wrinkles within the crow’s feet area with uneven and dull skin tone, lacking radiance and uniformity with regular skin texture and visible pores. Participants also agreed to avoid sun exposure during the study, and they signed and dated consent forms. Subjects were excluded from the study if they had a history of skin irritations or allergies to similar skin care products, foods, jewelry, or chemical products. Subjects were also excluded if they had a history of eczema, acne, dermatitis, psoriasis, or other severe skin abnormalities on the area being tested; experience prolonged sun exposure; utilize tanning beds or self-tanning products; use tobacco, drugs, or alcohol; or refuse to use only the products provided during their regular skincare routine, with the exception of their regular makeup products.
The selection of the study participants was overseen by Evalulab in Montreal, Canada. The decision to select the participants was based on the need to evaluate moderate wrinkles, and the development of wrinkles and crow’s feet is a common concern among women over the age of 30 years old. All clinical trial participants were informed both verbally and in writing about the nature of the test and potential risks. All volunteers read, signed, and dated the informed consent and understood the risk involved. The study design was approved by the review board of Evalulab, and written informed consent was obtained from all subjects participating in the trial.
Participants were instructed to use three products: a serum containing 10% apigenin to be applied over the face, a moisturizer, and an eye cream (both of which contain 10% apigenin). Patients were instructed to use the serum on the face avoiding the eye contour area but including the crow’s feet area. The serum penetrated for 30 to 45 seconds. Then the patient applied the moisturizer evenly on the face, avoiding the eye contour but including the crow’s feet area. The eye cream was applied evenly to the eye contour area including the eyelids. The use of all other skincare products except for mild cleansing and non-exfoliating cleansing products was prohibited during the study.
The study evaluations were conducted in a laboratory with controlled temperature and humidity on day zero, 28, and 56. We used a Visia-CR digital camera (supplied by Evaluab) to photograph participants’ faces. We also recorded the following measurements: skin elasticity via Cutometer, skin barrier function via Tewameter, skin hydration via Corneometer, skin luminosity via Glossymeter, and profilometry via silicon imprints of the wrinkles. Photographs and measurements performed at day zero were repeated on the second and third visit (study days 28 and 56). Silicon imprints of the face of the skin and the crow’s feet area were performed on all visits. Study participants were also encouraged to record observations in daily logs. On the day 28 and day 56 visits, participants had to return their completed daily logs as well as sample containers with unused products. Participants completed an electronic self-evaluation questionnaire on the final visit (day 56).
On study days 0 (baseline), 28 (midpoint), and 56 (final day), we evaluated the subjects’ skin for wrinkle size, depth, and number (crow’s feet) via profilometry. The crow’s feet area and facial wrinkles were differentiated by depth classes. Class I is defined as a depth of 0 to 55 µm (fine lines and fine wrinkles). Class II is defined as a wrinkle depth of 55 to 110 µm (moderate wrinkles), and Class III is a depth of 110 to 800 µm (deep wrinkles).
We evaluated skin roughness with profilometry and Quantilines software (Monaderm, Monaco). This software helps convert skin topography from the silicon imprints to skin roughness parameters. Skin elasticity was evaluated via cutometer. Skin hydration was measured using a Corneometer, and TEWL and skin barrier function were assessed with a Tewameter. We measured the subjects’ perception of the cream via self-evaluation questionnaires. The participants also completed self-report questionnaires on the product’s efficacy and sensory attributes.
Statistical analysis was completed on profilometry, skin roughness, skin hydration, skin barrier function, skin luminosity, skin elasticity, and skin tone evenness. We used a paired t-test to specifically compare the skin’s characteristics on study days 0, 28, and 56.
Results
Results obtained on study day 28 (after four weeks of treatment) and study day 56 (after eight weeks of treatment) were compared to baseline data (from day 0) using the paired t-Test. When appropriate, the results were expressed as the mean of the results of all participants.
Skin elasticity, firmness, tone
Ur/Ue represents firmness or net elasticity. This parameter decreases with aging and is considered the most important parameter in a cutaneous elasticity study. Statistical analysis or our results did not demonstrate any significant change in firmness on day 28 of the treatment regimen. Ur corresponds to tonicity or skin recovery and deformation. An increase in tonicity is associated with an increase in skin elasticity. No statistically significant changes were noted on day 28 of the treatment regimen. Uf represents the maximal amplitude or maximal deformation of the skin, a parameter that increases with aging. The average improvement observed for this parameter (-3%) was not statistically significant. Ue represents the immediate extensibility or ease of deformation of the skin. A reduction in Ue corresponds to an improvement in firmness measured by skin resistance to deformation. In this study, the Ue parameter did not show any Skin elasticity is a combination of the following skin parameters: Of (maximal amplitude), Ur (tonicity), Ue (immediate extensibility), and Ur/Ue (firmness or net elasticity). The summary of the changes in skin elasticity at baseline, day 28, and day 56 is provided in figures 1 and 2.
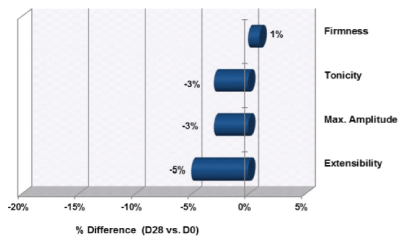
Figure 1: Changes in Skin Elasticity from D0 to Day 28 (Uf, Ur, Ue, and Ur/Ue)
Note: Increases in firmness (Ur/Ue) and tonicity (Ur) are associated with improved skin elasticity. Reductions in extensibility (Ue) and max amplitude (Uf) are associated with improved skin elasticity.
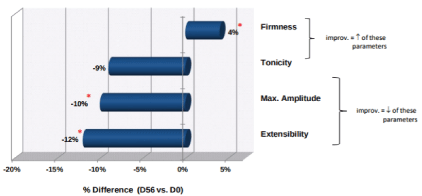
Figure 2: Changes in Skin Elasticity from D0 to Day 56 (Uf, Ur, Ue, and Ur/Ue)
*p<0.05
For an improvement in skin elasticity, the firmness (Ur/ Ue) and tonicity (Ur) parameters need to increase, whereas maximum amplitude (Uf) and extensibility (Ue) need to decrease.
By study day 56, there was a significant change in firmness, maximum amplitude, and extensibility (Figure 2). Firmness or net elasticity increased significantly by 4% (p<0.05), with a maximum improvement of 41%. The mean maximum amplitude decreased significantly by 10% (p<0.05) with effectiveness at -54%. Extensibility also decreased significantly by 12% (p<0.05) with percentages down to 60%. The mean tonicity factor decreased by 9%, but this was not enough of a change to be considered statistically significant. Overall, after eight weeks of treatment, changes were observed in firmness, maximum amplitude, and extensibility, which represent an overall improvement in skin elasticity.
Significant change on day 56 of treatment (Figure 2). As stated above this change in elasticity was not statistically significant at day 28 but was at day 56.
Tolerability
All participants completed the study in its entirety, and no extraordinary reactions were reported. There were no adverse effects reported by the participants or observed throughout the course of this study. Tolerance data were recorded individually for each of the three products in a self-report questionnaire by participants on study day 28 and study day 56. All other aspects of the skin care regimen were evaluated together, as they were applied by the participants twice daily as part of their beauty routine. The results of the sensory attribute data collected are presented in tables 1-3. We used version 4.03 of CTCAE to assess the severity of the adverse reactions.
| Intolerance Criteria |
None |
Slight |
Moderate & High |
| Stinging |
Day 28 |
Day 56 |
Day 28 |
Day 56 |
Day 28 |
Day 56 |
| Burning Sensation |
100% |
100% |
0% |
0% |
0% |
0% |
| Burning Sensation |
100% |
100% |
0% |
0% |
0% |
0% |
| Eye Watering |
95% |
100% |
5% |
0% |
0% |
0% |
| Tightness |
90% |
86% |
5% |
14% |
5% |
0% |
| Redness |
81% |
85% |
19% |
5% |
0% |
0% |
Table 1: Tolerance to “Smart C Serum” at Day 28 and Day 56
| Intolerance Criteria |
None |
Slight |
Moderate & High |
| |
Day 28 |
Day 56 |
Day 28 |
Day 56 |
Day 28 |
Day 56 |
| Stinging |
100% |
90% |
0% |
10% |
0% |
0% |
| Tightness |
100% |
100% |
0% |
0% |
0% |
0% |
| Puffiness |
100% |
95% |
0% |
5% |
0% |
0% |
| Redness |
95% |
95% |
5% |
0% |
0% |
5% |
| Burning Sensation |
85% |
81% |
10% |
5% |
5% |
5% |
| Eye Watering |
81% |
72% |
19% |
14% |
0% |
5% |
| Ocular Discomfort |
76% |
76% |
19% |
19% |
5% |
5% |
Table 2: Tolerance to “Eyejuvenate Apigenin Eye Treatment” at Day 28 and Day 56
| Intolerance Criteria |
None |
Slight |
Moderate & High |
| |
Day 28 |
Day 56 |
Day 28 |
Day 56 |
Day 28 |
Day 56 |
| Burning Sensation |
100% |
100% |
0% |
0% |
0% |
0% |
| Eye Watering |
100% |
100% |
0% |
0% |
0% |
0% |
| Stinging |
95% |
95% |
5% |
0% |
0% |
5% |
| Tightness |
95% |
90% |
5% |
5% |
0% |
5% |
| Redness |
90% |
95% |
5% |
5% |
5% |
5% |
Table 3: Tolerance to “Skintelligent Apigenin Hydration Skin Repair” at Day 28 and Day 56
Hydration
The skin hydration data are provided in table 4. Hydration levels were stable but did not improve significantly by days 28 and 56.
| Skin Hydration |
D0 |
Day 28 |
% Difference |
Significance |
Day 56 |
% Difference |
Significance |
| Average |
48.5 |
49.6 |
2% |
NS |
49.2 |
1% |
NS |
Table 4: Changes in Skin Hydration from D0 at Day 28 and Day 56
Barrier function and transepidermal water loss
A summary of the changes in the skin barrier function at study day 0, 28, and 56 are presented in table 5. The data represent the averages of the different sites, combined for all study participants. Skin barrier properties were not statistically significant on day 28 and day 56 of product use. The baseline average was low (9.9), indicating study participants had good skin barrier properties at the start of the study. The average value of TEWL remained low throughout the study, which suggests that the study treatment did affect skin barrier function.
| Skin Barrier |
D0 |
Day 28 |
% Difference |
Significance |
Day 56 |
% Difference |
Significance |
| Average |
9.9 |
10.1 |
2% |
NS |
9.9 |
0% |
NS |
Table 5: Changes in Skin Barrier Function from D0 to Day 28 and Day 56
NS=Not Significant
Eye wrinkle length and depth
Assessment of wrinkle size, depth, and number (i.e., crow’s feet) were evaluated using profilometry. A summary of profilometry measurements expressed as number of wrinkles, area of wrinkled skin, total length, mean length and mean depth of wrinkles is presented in table 6. The data provided for each parameter correspond to the average obtained for the all participants on day zero, day 28, and day 56.
| Profilometry Results |
D0 |
Day 28 |
% Difference |
Significance |
Day 56 |
% Difference |
Significance |
| Number of Wrinkles |
296 |
287 |
-3% |
NS |
277 |
-6% |
NS |
| Area (mm2) |
21.44 |
20.82 |
-3% |
NS |
19.99 |
-7% |
NS |
| Total Length (mm) |
188.75 |
179.24 |
-5% |
NS |
179.36 |
-5% |
NS |
| Mean Length (mm) |
0.63 |
0.61 |
-3% |
NS |
0.62 |
-2% |
NS |
| Mean Depth (mm) |
55.21 |
54.23 |
-2% |
NS |
54.88 |
-1% |
NS |
Table 6: Profilometry Findings: Changes in Skin Wrinkles (Crow’s Feet Areas) from D0 to Day 28 and Day 56
NS=Not Significant
While we noted a reduction in all parameters, we found no statistically significant variance on study day 28 and day 56 when compared to the baseline results on study day zero. The anti-wrinkle effect of the tested regimen was further evaluated by classifying wrinkles into three classes:
- Fine lines and wrinkles with depth from 0 µm to 55 µm
- Moderate wrinkles with depth from 55 µm to 110 µm
- Deep wrinkles with depth from 110 µm to 800 µm
The results obtained for profilometry depth classes are summarized in figure 3 for the number of wrinkles in each class and figure 4 for the change in depths in each class. The data revealed after 28 days; there was a favorable tendency in the number of Class II wrinkles (p<0.1). There was a significant decrease of 2% in Class I wrinkles with depths from 0 to 55 µm (i.e., fine lines). The maximum reduction of Class I wrinkles was 10%. There was no significant change in the depth of Class II or Class III wrinkles. After 28 days, there was no change in the number of Class I or Class III wrinkles. After 56 days, there was no significant change in the number of Class I, II, or III wrinkles. At 56 days, there was no significant change in the depth of wrinkles of Class I, II, or III wrinkles. Figure 5 is a sample representation of the profilometry images from the same participant at day 0, day 28, and day 56.
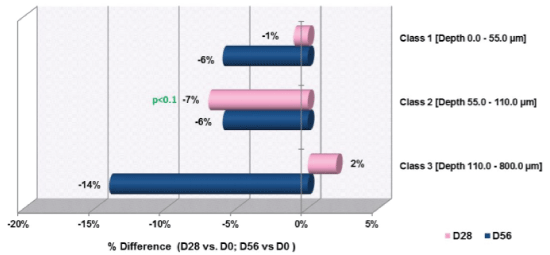
Figure 3: Changes in Number of Wrinkles by Class from D0 to Day 28 and Day 56
*p<0.1=favorable tendency
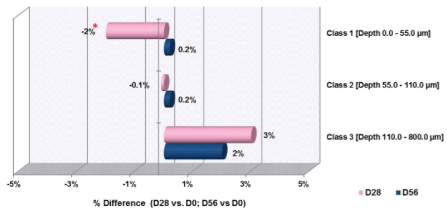
Figure 4: Changes in Wrinkle Depth by Class from D0 to Day 38 and Day
56
*=p<0.05
Figure 5: Profilometry Images at Day 0, Day 28, and Day 56
kin luminosity
Skin luminosity data was evaluated using a glossymeter. The data regarding the changes in skin luminosity from baseline (D0) to day 28 and day 56 are presented in table 7. Skin luminosity was not improved from baseline at either day 28 or day 56. Statistical analysis did not show a significant change in the average values.
| Skin Gloss |
D0 |
Day 28 |
% Difference |
Significance |
Day 56 |
% Difference |
Significance |
| Average |
5.4 |
5.2 |
-3% |
NS |
5.1 |
-5% |
NS |
Table 7: Changes in Skin Luminosity from D0 to Day 28 and Day 56
NS=Not Significant
Skin tone evenness
Image analysis was conducted to evaluate the changes in skin tone evenness from baseline (D0) to study day 28 and day 56. Digital photographs were obtained using the Visia-CR for evaluation. These data are presented in table 8. Skin tone evenness was not improved after 28 or 56 days of treatment.
| Color Distance |
D0 |
Day 28 |
% Difference |
Significance |
Day 56 |
% Difference |
Significance |
| Average |
56.5 |
58.2 |
3% |
NS |
56.9 |
1% |
NS |
Table 8: Changes in Skin Tone Evenness from D0 to Day 28 and Day 56
NS=Not Significant
Skin roughness
Skin roughness and texture were evaluated using profilometry and the use of Quantilines software. The volume parameters for roughness are presented in table 9 and figure 6 and are expressed as the average for the group of participants. The volume parameters of roughness at day 28 was significantly reduced by 5% (p<0.05), as shown in figure 6. There was also a reduction of 2% at day 56, but this change was not statistically significant. The average Skin Roughness (Rz) was not improved at the day 28 or 56 evaluations.
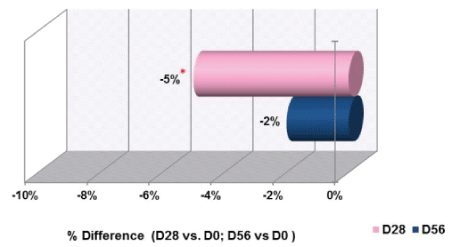
Figure 6: Changes in Skin Roughness Parameters When Comparing Day 28 and Day 56 to D0
Note: An improvement in skin roughness results in a decrease in the average and volume of roughness.
| Color Distance |
D0 |
Day 28 |
% Difference |
Significance |
Day 56 |
% Difference |
Significance |
| Average Skin Roughness (Rz) |
111.5 |
109.7 |
-2% |
NS |
110.1 |
-1% |
NS |
| “Volume” Parameter for Roughness |
152.9 |
145.4 |
-5% |
S |
149.9 |
-2% |
NS |
Table 9: Changes in Skin Roughness from D0 to Day 28 and Day 56
Statistical Significance: S=p<0.05; NS=Not Significant
Note: An improvement in skin roughness results in a decrease in the average and volume of roughness
Perception
Participant perception data are provided in table 10. By the end of the treatment period, most participants reported their skin felt more refined, was less tired, more hydrated, fresher looking, softer, firmer, more elastic, and brighter. A majority of participants also reported fewer visible wrinkles around the eyes, and fewer visible pores. A minority reported attenuated brown spots.
| After Day 56 of skin care routine... |
Total Positive Responses |
| My skin texture is more refined |
95% |
| My skin looks less tired |
95% |
| My skin is more hydrated |
90% |
| My skin is more soft and more supple |
90% |
| My skin is more fresh looking |
86% |
| My skin seems more firm and more elastic |
81% |
| My skin looks brighter and more even |
81% |
| Fine lines and wrinkles around my eyes are less visible |
76% |
| Pores are less visible, dilated, and tightened |
71% |
| The products help to fight the signs of aging |
71% |
| My skin looks younger |
67% |
| Fine lines and wrinkles on my face are less visible |
62% |
| Dark circles under my eyes are less visible |
53% |
| Puffiness under my eyes is less visible |
50% |
| Brown spots (age spots) are attenuated |
44% |
Table 10: Participant-Reported Perception of Test Regimen on Day 56
Sensory attributes
Sensory attribute data are presented in tables 11-13. The sensory attributes for the “Smart C Serum” were very well received by the participants with a score of 76 to 95%, the color and ease of application receiving the highest scores at 95%. The sensory qualities of the “Eyejuvenate Apigenin Eye Treatment” were also well received by the participants. The scores ranged from 81% to 95%, with texture and ease of application receiving the highest scores of 95% satisfaction. The “Skintelligent Apigenin Hydration Skin Repair Cream” was also well received by participants. Scores ranged from 81% to 100% satisfaction, with absorption and ease of application being the highest scores of 100%.
| Criteria |
Total Appreciated |
| Color |
95% |
| Ease of Application |
95% |
| Absorption After Application |
86% |
| Comfortable Feeling After Application |
86% |
| Odor |
81% |
| Texture |
76% |
| Hydrating Effect After Application |
76% |
Table 11: Overall Scores for Sensory Attributes of “Smart C Serum”
| Criteria |
Total Appreciated |
| Texture |
95% |
| Ease of Application |
95% |
| Absorption After Application |
90% |
| Hydrating Effect After Application |
90% |
| Color |
86% |
| Comfortable Feeling After Application |
86% |
| Odor |
81% |
Table 12: Overall Scores for Sensory Attributes of “Eyejuvenate Apigenin Eye Treatment”
| Criteria |
Total Appreciated |
| Ease of Application |
100% |
| Absorption After Application |
100% |
| Texture |
90% |
| Comfortable Feeling After Application |
90% |
| Hydrating Effect After Application |
90% |
| Color |
81% |
| Odor |
81% |
Table 13: Overall Scores for Sensory Attributes of “Skintelligent Apigenin Hydration Skin Repair”
Discussion
In recent years, there has been increased focus on natural agents which possess antioxidant and anti-inflammatory properties for use in skin care products [16,17]. There are several reasons for this recent trend, as these bioactive agents derived from food sources are safe and may provide additional value upon dermal application [18,19]. Apigenin’s anti-aging properties are based in its ability to increase the expression of COX-2, a critical mediator of inflammation and angiogenesis [10]. Additionally, apigenin decreases the production and activity of proteases including MMP-1 and MMP-2, which could also potentially hinder angiogenesis [20]. One study found that when apigenin was applied topically, it enhanced dermal thickness and increased skin elasticity [14]. Hou M et al. [2] demonstrate that topical apigenin significantly enhanced permeability barrier homeostasis, indicating apigenin may have the potential to assist in skin rejuvenation.
Ultraviolet (UV) radiation causes significant skin changes, including the degrading of collagen, which is likely mediated by MMPs [21]. According to Choi S et al. [14] apigenin inhibits the UVA-induced induction of MMP-1 expression, which may slow the degradation of the collagen matrix.
According to Zhang Y et al. [5] a “decline in the production of collagen in aging fibroblasts is mainly responsible for decreasing dermal thickness seen in extrinsically aging skin, which reveals dermal atrophy, fragmentation, and irregular collagen bundles”. As a result, researchers looked for ways to increase the dermal thickness and collagen density of the skin. One study reported apigenin increased dermal thickness and collagen density in test subjects [7].
While the day zero evaluation served as the control, the study was somewhat limited due to the small sample size. In addition, there was no inclusion of a placebo or blinding, which may have provided additional information regarding the effectiveness of the skin care regimen and potential participant bias.
Conclusions
Our findings support the evidence that apigenin, a wellknown antioxidant with anti-inflammatory effects, can improve several markers of aging such as firmness, elasticity and fine wrinkling and maintains of hydration. Apigenin use in topical products may contribute to objectively improved parameters of skin health and subjective appearance of photo-aged skin. Further studies are warranted to validate this conclusion. Under the conditions of this study, the anti-aging regimen developed was well tolerated and well appreciated by the volunteers for various subjective parameters such as sensory attributes and improvement of overall skin condition.
Analysis of the data demonstrates that the test regimen did not compromise skin barrier function. Furthermore, the test treatment provided statistically significant improvements in skin roughness for the “volume” parameter and in the depth of fine lines and wrinkles Class 1 after 28 days of use only. Finally, significant improvements were measured in skin elasticity for the firmness, maximal amplitude and extensibility parameters after 56 days of treatment. The test regimen may be considered as having a significant effect on skin elasticity.
Acknowledgements
We would like to thank all the individuals who helped with the experiments and made other contributions. This study was conducted by Evaluate (Montréal), a contract research organization, and support for the study was funded by EA Beauty.
Conflict of Interest
This research is sponsored by EA Beauty and may lead to the development of products, in which the authors have a business and/or financial interest.







