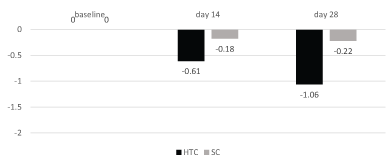
Figure 1: Reduction of thigh circumference (cm)


Massimo Milani1* Mario Puviani2
1Difa Cooper Medical Department Caronno Pertusella (VA), Italy*Corresponding author: Massimo Milani, Medical Director, Difa Cooper Medical Department Caronno Pertusella (VA), Via Milano 160, Italy, E-mail: Massimo.Milani@difacooper.com
Introduction: Cellulite (gynoid lipodystrophy) is a common skin alteration representing mainly a cosmetic concern rather than a pathological condition. Cellulite in different stages of severity affects up to 98% of post pubertal women. The initial phase of cellulite is characterized by subdermal tissue oedema with fluids retention. A new topical product with draining action (HTC) containing escine, beta-sytosterols, caffeine and hypertonic sodium chloride has been recently developed. In an experimental human skin model study, HTC has demonstrated to be able to drain from the skin a water amount of 5% of its weight when applied over the skin specimen.
Study aim: To evaluate the clinical efficacy of HCT in the clinical improvement of cellulite in comparison with placebo (vehicle cream) (VC). .
Subjects and methods: In a randomized, double blind, intra-patient (right vs. left) controlled trial a total of 30 women (mean age 34 years) with Grade I or II cellulite were enrolled after their informed consent. HTC and the placebo cream were applied randomly once daily on the right or left site of lower limb in a double-blind fashion for 28 days. Primary outcomes of the trial were the evolution of thigh circumference measurements, evaluated at baseline, day 14 and day 28, ultrasonography assessment of adipose panniculus and elastometry, evaluated at baseline and after 28 days of treatment. Secondary outcomes were the 5-point Investigator and Patients global efficacy assessments (IGE and PGE) score (from 0 to 4) of orange peel appearance.
Results: In the HCT treated sites thigh circumference was reduced significantly (p=0.003) by -0.61 cm and by -1.06 cm, after 14 and 28 days of treatment, respectively, in comparison with baseline value. VC application reduced thigh circumference by -0.19 cm (day 14) and -0.23 cm (day 28): this difference was not statistically significant in comparison with baseline. Ultrasonography assessment of adipose tissue showed a significant (p=0.05) reduction (-1.73 mm after 28 days) after HTC application in comparison with baseline. In the VC treated site ultrasonography assessment showed a non-significant reduction in comparison with baseline of -0.77 mm after 28 days, respectively. Elastometry in HCT improved by 1.4% in HCT and by 0.89% in the VC. IGE assessment of orange peel appearance showed an improvement (without pinch test) in 33% in HCT and 7% in VC (p=0.02 between treatments) and in 40% in HTC and 20% in VC (after pinch test) (p=0.05, between treatments). At baseline the no-pinch IGE score was 2.3 in both HCT and VC groups. At day 28, no-pinch IGE score significantly (p=0.0019) decreased to 1.9 only in HCT treated sites. In VC the no-pinch IGE score was 2.2 at day 28. PGE evaluation of orange peel appearance showed that 73% of subjects noted a significant improvement in HTC treated sides vs. 37% in the VC treated sides (p=0.004). Both products were well tolerated.
Conclusion: This new HTC has shown a superior clinical efficacy in comparison with the vehicle cream in improving both objective and subjective assessments of cellulite parameters.
Lipodystrophy; Randomized controlled trial
Cellulite (gynoid lipodystrophy) is a common skin alteration representing mainly a cosmetic concern rather than a pathological condition [1]. Cellulite in different grade of severity affects 85%-98% of post pubertal women [2,3]. Cellulite is also defined as a localized metabolic disorder mainly affecting the subcutaneous tissue [1]. Cellulite commonly affects pelvic region, lower limbs and abdomen [1]. This alteration therefore can modify the body shape [1]. The initial phase of cellulite is characterized by subdermal tissue oedema [1] with fluids retention [1]. Topical pharmacological treatment of cellulite could be problematic [1]. A new hypertonic topical product in cream formulation with draining action (HTC) (Dermolipid Aqua®, Difa Cooper, Caronno Pertusella, Italy) containing escine, beta-sytosterol, caffeine and hypertonic sodium chloride has been recently developed. When applied on the skin, the product activates an osmotic process which releases the liquids accumulated between cells, thus allowing subsequent removal. In an experimental human skin model study, HTC has demonstrated to be able to drain from the skin a water amount of 5% of its weight when applied over the skin specimen in comparison with an isotonic cream vehicle [1].
We performed a right vs. left, double-blind, randomized controlled trial to evaluate the clinical efficacy of HCT in the clinical improvement of cellulite in comparison with a placebo cream (VC). The VC was the vehicle cream without active components.
Thirty women (mean age 34 years) with cellulite of Grade I and II in severity (according to Rossi et al. [11]) were enrolled after their informed consent. The study followed the Helsinki guidelines for clinical trials. This study protocol was approved by the local Research Ethics Committee and all subjects signed informed consent forms Study took place between January 2013 and December 2014. Eligible participants were healthy adult women, aged 18–50 years with a BMI <29.9 kg/m2 , with regular menstrual cycle, and presence of cellulite of gluteal and posterior thigh bilaterally. Enrolled subjects wore only underwear without shoes when assessing weight and height. Subjects were excluded if they were in aesthetic treatments or treatments in the gluteal region and thighs in the previous 2 months before the start of this study. Pregnancy or a recent pregnancy (<6 months), cardiovascular problems, metabolic disorders, diabetes mellitus, immunosuppression, and skin lesions at the treatment sites were defined as other exclusion criteria. Enrolled women were instructed to apply every day both products in a random fashion according to a randomization list (right vs. left site) by a slight massage over the lower limb (thigh and buttock) using 5 g of product for each application. Randomization list was generated by a dedicated computer program. During the duration of the study enrolled women were also instructed to follow the same exercise and eating habits of the period before the enrollment.
Primary outcomes of the trial were the evolution of thigh circumferential measurements, ultrasonography assessment of adipose panniculus and elastometry, before and after 14 and 28 days of treatment. Secondary outcomes were Investigator and Patients global efficacy assessments (IGE and PGE) of orange peel appearance using a 5-point score from 0 to 4. IGE score evaluation was performed with or without pinch test. Thighs circumference measurements were obtained using a measuring tape, with the volunteers standing in a standardized upright position and performing the measurement up to 18 cm of knee. Ultrasonography was performed at the trochanteric region level of the thigh using 7 Mhz device (Logic alfa100 GE Medical). Elastometry evaluation was performed using Cutometer MPAA580 device. Clinical evaluation of cellulite was assessed using a 5-point scale grade (from 0: no cellulite to 4: severe cellulite) with or without pinch test. The two products (HTC and VC) were supplied as creams of similar appearance and texture. All the samples were blindly assigned a code, before being stored at ambient humidity and temperature, in their original container. HTC and the comparator were applied once daily on the right or left site in a double-blind fashion for 28 days.
Statistical analysis was performed using SPSS statistical software ver. 12.0. Continuous variables were expresses as mean ± Standard Deviation (SD). The primary endpoint of the trial was the evolution of thigh circumference from baseline and after 28 day of treatment. The paired T test and the Wilcoxon test were used for the evaluation of the variables during the study (baseline, day 14 and day 28). A secondary endpoint was the percentage of improvement of orange peel appearance evaluated by IGE and PGE. For these variables, we calculated the 95% Confidence intervals. According to the exploratory nature of the present study a formal sample size calculation would not be considered mandatory. However, we planned to treat at least 30 women having in this manner 30+30 variables values.
All 30 enrolled subjects completed the study. In the HCT treated sites thigh circumference was reduced significantly (p=0.003, paired T test) by -0.61 cm and by -1.06 cm, after 14 and 28 days of treatment, respectively, in comparison with baseline value. VC application reduced thigh circumference by -0.18 cm (day 14) and -0.22 cm (day 28): this difference was not statistically significant in comparison with baseline (p=0.2) (Figure 1). At the end of 28 days of treatment thigh circumference in the HCT group was 0.7 cm less than the thigh circumference in the VC group (60.8 cm vs. 61.5; P=0.0001; WIlcoxon Paired t test). Ultrasonography assessment of adipose tissue showed a significant (P=0.05; paired T Test) reduction (-1.1 mm after 14 days and -1.73 mm after 28 days) after HTC application in comparison with baseline. In the VC treated site ultrasonography assessment showed a non-significant reduction of -0.43 mm and -0.77 mm after 14 and 28 days, respectively, in comparison with baseline. Elastometry assessment improved by 1.4% in HCT and by 0.89% in the VC (Figure 2). IGE assessment of orange peel appearance showed an improvement (without pitch test) in 33% in HCT and 7% in VC (p=0.02; Chi-square test) and in 40% in HTC and 20% in VC (after pitch test) (p=0.05; Chi-square test). At baseline the no-pitch IGE score was 2.3 in both HCT and VC groups. At day 28, no-pitch IGE score significantly (p=0.0019) decreased to 1.9 only in HCT treated sites. In VC group the no-pitch IGE score was 2.2 at day 28. PGE evaluation of orange peel appearance showed that 73% of subjects noted a significant improvement in HTC treated sides vs. 37% in the VC treated sides (p=0.004; Chi-square test).

Figure 1: Reduction of thigh circumference (cm)
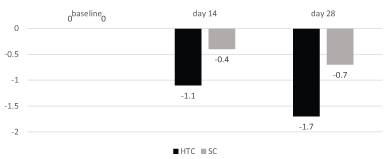
Figure 2: Ultrasound assessement of adipose tissue thickness (mm)
Our study has shown that a new hypertonic cream with draining effect is effective in the clinical improvement of mild to moderate cellulite. Cellulite is considered a non-pathological skin disorder with structural changes at the level of dermis and hypodermis [12]. The term cellulite commonly refers to dimpling of the skin of the thighs and buttocks [13]. This condition, mainly in the early phases, can cause the retain of water at the adipocytes level [14]. This process induces a dramatic increase in adipocyte volume. The increased volume of adipocytes eventually induces the characteristic modifications of hypodermis structure with the classical formation of orange peel appearance [7]. The HTC evaluated in this study has previously demonstrate in in vitro human skin model to be able to drain from the skin a water amount of 5% of its weight when applied over the skin specimen. This draining effect could be useful in the treatment of early stage of cellulite. The tested cream contains escine, beta-sytosterol, caffeine and hypertonic sodium chloride. When applied on the skin, this emulsion activates an osmotic process which releases the liquids accumulated between cells, thus allowing subsequent elimination through the skin. In the present study, the clinical effect of HTC was greater than the vehicle product. A superior effect was observed both in objective measurements (i.e. thigh circumference, elastometry and ultrasonography) and subjective evaluations (i.e. clinical improvement score). In the present study thigh circumference reduction in the HCT group was -1.06 cm in comparison with baseline. This reduction seems higher than the reduction obtained with other anti-cellulite treatments [15] like radiofrequency energy, infrared light, and mechanical manipulation. Some limitations should be taken in account in evaluating our results. First, we have compared the efficacy of HTC with a placebo cream (VC). However, the exploratory nature of the present trial was to evaluate the efficacy of the product. Future comparative explanatory studies are warranted to evaluate the efficacy in comparison with anticellulite standard topical products. A second issue was that we did not take note during the study visits of the subjects’ menstrual cycle phase. It is well known that cellulite could be influenced and aggravated by cyclic edema [16]. However, in consideration of the fact that our study was an intra-patient evaluation this potential confounding factor could have influenced the effect of both study product in the same way.
We have demonstrated that this new hypertonic topical product containing escine, beta sitosterol and caffeine has a superior clinical efficacy in comparison with vehicle cream in improving both objective and subjective assessments of cellulite parameters.
The study was supported by a grant of Difa Cooper SpA.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Milani M, Puviani M (2017) Clinical Efficacy of a New Hypertonic Draining Cream in the Treatment of Cellulite: A Randomized, Double Blind Right-vsLeft Placebo-Controlled Trial. J Clin Cosmet Dermatol 1(2): doi http://dx.doi.org/10.16966/2576-2826.109
Copyright: © 2017 Milani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access