Abstract
Background and aim: Investigations of new possibilities for oesophagus replacement remains a challenge in digestive surgery. In the present study combined prosthesis with artificial and biological components proposed for oesophagus repair were tested by imaging techniques.
Methods: Experiments were conducted on 30 laboratory rats which have survived a reconstructive surgery of the oesophagus. A bio prosthesis made of a degradable chitosan tubular scaffold and a vascularized flap of locally grown foetal oesophagus-stomach implant or of a local muscle were used for bridging oval or circular defects of the cervical oesophagus. Before the main reconstructive operation, ultra sound was used for evaluation of the growing foetal implant and its suitability for reinforcement of the prosthesis. Success of the reconstruction was assessed by both functional and morphological investigations. Roentgen imaging was used to assess the prosthesis presence and degradation delays, on the one hand, and the alimentary bolus transit through the reconstructed segment, on the other. After that, the animals were euthanized and histological examination of the reconstructed oesophagus was provided. Investigations were performed on the animals from 1week to 13 months after operation.
Results: The results were encouraging: chitosan has shown adequate properties as a scaffold for both oval and circular defect bridging. After adjustments of operation techniques and follow up methods, the surgery could be considered as successful with a satisfying survival percent, a complete animal general condition recovery and the absence of leakage or significant stenosis. In vivo obtained images corresponded to the histologic findings of the reconstructed oesophagus wall restoration.
Conclusion: Roentgen and ultra sound imaging (static and serial images) is possible and adequate to assess the presence of grown foetal oesophageal cyst necessary for the oesophagus defect repair, to verify the prosthesis evolution and the integrity or damage of the oesophagus after reconstructive surgery in the rat.
Keywords
Bio Prosthesis; Chitosan Scaffold; Combined Prosthesis; Oesophagus Defect Repair; Roentgen and Ultrasound Imaging in Small Animals
Abbreviation
BW-Body Weight
Introduction
Imaging in medicine has reached fantastic levels nowadays, when molecular processes are visualized [1-5] Nevertheless, macroscopic imaging is still necessary and provided in order to test function, particularly transit function, of different digestive organs in pathologic situations or after correction of defects, not only in human, but also in small laboratory animals. In fact, it is not possible yet to avoid the necessary step of animal experimentation especially in reconstructive surgery [6]. The oesophagus repair remains a tremendous subject of such a surgery. In fact, since decades the methods and results of oesophagus major surgery are stagnant [7-14] and the need of new possibilities is great. In the 20th century 50-60-ties the use of rigid waterproof non degradable tubes prosthesis was tried but it has led to failures: either sliding of the prosthesis towards the stomach, or erosions and ulcers development followed by abscess or stricture [15-17]. As it was already predicted in the last century 60-70-ties [16], the key of the success might be the combination of a waterproof but degradable support-scaffold with a biologic vascularised reinforcement as a basis for tissue repair. But such material for tubes was not yet accessible. During the past years, new methods of hollow organ reconstruction were proposed based on tissue engineering. Some success was registered with trachea reconstruction, but up to now similar attempts in oesophagus reconstruction remain experimental [18-25]. In our work [20,22] chitosan, a degradable polymer derived from mushroom chitin [26-27] was proposed as a tubular scaffold to support a repaired oesophagus segment. One of the crucial moments was the follow up of both prosthesis degradation and reconstructed organ function and morphology. We have tried to adapt the old methods of classic Roentgen and ultra sound investigation to our purpose.
Material and Methods
Experiments were conducted on 30 rats Wistar and Fischer strains, both sexes, BW 200-400 g.
They included 5 intact animals as a control, 5 animals with chitosan tubes implanted between the muscles of the neck close by the oesophagus, 5 animals with chitosan tube fixed within intact oesophagus, 15 animals after successful repair of oval (5) and circular (10) defects of the cervical oesophagus. (Table 1) Investigation of the chitosan bio compatibility and resorption in vivo was provided in 5 rats: after longitudinal incision of the anterior neck skin, the tube used for oesophagus stenting (Diameter 2 mm, length 15 mm) was placed between the neck muscles. The incision was sutured by isolated stiches (Vicryl ® 4°°). Roentgen control was performed once a week during 2 months. Investigation of the chitosan tube degradation after introduction into cervical oesophagus by intubation technique and fixed within the oesophagus, was provided in 5 rats. Roentgen control was performed 1 week after its insertion. For visualization of the chitosan tubes out and within the oesophagus a digitalized table Roentgen (General Electric’s “Prestige” NH, USA) was used. After that, the animals were euthanized (by anaesthetics overdose) and necropsy was performed to verify the imaging results.
| Series |
Animal number |
Observation delay |
Remarks |
| Control 1: intact rats |
5 |
- |
unique investigation |
| Control 2: chitosan tubes implanted under the neck skin |
5 |
up to 2 months |
repeated investigations |
| Control 3: chitosan tubes within intact oesophagus |
5 |
up to 2 months |
repeated investigation |
| Repair of oval oesophagus defects |
5 |
up to 10 months |
repeated investigation |
| Repair of circular oesophagus defects |
10 |
up to 13 months |
repeated investigation |
| TOTAL |
30 |
from 2 to 13 months |
|
Table 1: Series and animal number
Ultra sound investigation (Siemens with a 17 MHx probe, Germany) was performed to identify and measure the cyst, developing after the implantation of a foetal oesophagusstomach segment of about 5 × 5 × 2 mm3 under the skin, between the muscles of the neck not far from the oesophagus. It was supposed to confirm the possibility of using a flap of the grown implant to enhance the oesophagus lesion repair. Three types of operations for creating and repairing oesophagus oval or circular defects were provided. Their schemas are presented in figure 1.
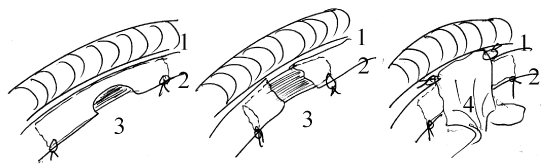
Figure 1: Schema of surgical techniques used for repair of cervical oesophagus defects
A. Oval defect, B. Circular defect with chitosan scaffold, C. Circular defect with chitosan tube and vascularized flap enforcement.
1. Trachea
2. Oesophagus
3. Chitosan tube
4. Vascularized flap (from grown foetal oesophagus implant or from neck muscle).
After Nembutal® anaesthesia (0.075 mg/kg BW) and subcutaneous injection of Atropine sulphate 1%-2ml, the neck part of the oesophagus was exposed, chitosan tube was introduced into oesophagus per mouth, fixed at its distal end to the oesophagus by 1-2 silk stiches (6°°). A part of the organ was resected around the tube and the edges were fixed by 4 silk stiches (6°°). The surface of oval defect was about 8 mm², the length of the resected segment varied between 4 mm and 6 mm length that is 1/3 of the length of the cervical portion of the oesophagus. In half of the cases a vascularized flap of the cyst, obtained from grown foetal oesophagus implant, was fixed on both edges of the oesophagus defect by 4 silk stiches (6°°). The wound was sutured with Vicryl® 4°° (See also [22]). The observation delays ran up to 13 months after operation. Investigation of the repaired oesophagus was performed in 15 animals having survived the operation within 2, 4,6 and 13 months. For investigation of the swallowing and transit functions of the oesophagus the animals received Nembutal® sedation and were maintained in elastic contention [28], so that the swallowing capacity could be conserved. A catheter size 16 fixed on a 10 ml syringe was introduced in the mouth between jaws and cheeks and a Gastrografin® solution was mildly injected till the stomach began to fill. Histological control examination of necropsy material after euthanasia (by anaesthetics overdosing) of these animals was provided with fixation by formalin 12%, embedding in paraffin, slices 4 mcm, staining haematoxylin eosin saffron. All the procedures, including imaging, were performed under anaesthesia ‘Fluorotane© 4% 1 min/100 g BW, Nembutal© 0.075 mg/100g BW and Temgesic© 0.2 ml intra peritoneum, Atropine sulphate 1% 0.2 ml by subcutaneous injection.
Animal management was conducted according to the Helsinki Rules and with approbation of the local Animal Ethics Committee (protocol 50N).
Results
Roentgen examination of the neck of animals with implanted chitosan tubes between tissues (pH about 7.0-7.4) has shown that these tubes may be visible even within 2 months after operation (Figure 2). They did not induced any significant reaction of their implantation site. The chitosan scaffold placed into the oesophagus lumen (pH < 6) was no longer visualized within 2-4 days. If alkalinisation of the oesophagus contents was provided (water feeding with pH 7.1-7.4), the chitosan degradation delay increased up to 9-12 days (Figure 3). Ultra sound investigation of the site of foetal oesophagusstomach implantation has allowed imaging and measuring of the growing organoid and determining of the proper moment for its use in oesophagus defect reinforcement, that is when its dimensions were no less than 1.5 × 1.3cm (Figure 4). In the cases of oesophagus defect repair, at day 7a thin waterproof fibrous membrane which had already formed around the chitosan scaffold, could ensure the continuity of the organ wall in spite of the scaffold degradation. This was confirmed by histologic examination (Figure 5). At months 4, 6, 10 and 13, the permeability of the repaired oesophagus was proved, with normal swallowing and peristalsis of the organ (Figure 6). In some animals a small diameter decrease -about 10-15% was noted. In some other cases a slight increase of the diameter (about 10%) could be observed. This corresponded to the macro and microscopic findings of complete repair of circular defects of the cervical oesophagus with genuine restoration of the wall structure in these just examined animals (Figure 7).
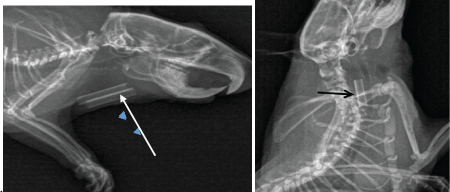
Figure 2: Roentgenograms of chitosan tubes (arrow) into the neck tissues 2 months after implantation
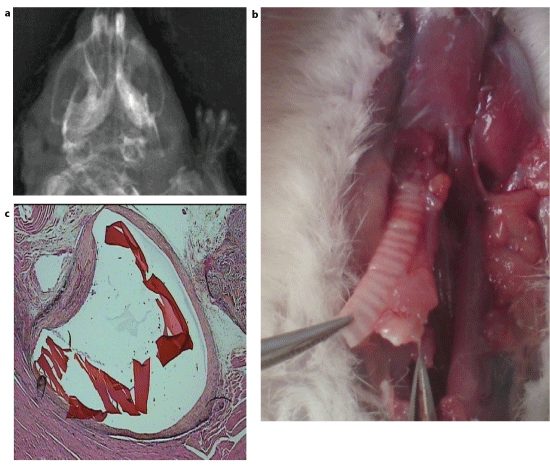
Figure 3: Degradation of a chitosan tube in the intact oesophagus.
a. Roentgen picture of the tube at day 9 (arrow).
b. Macroscopic view the same day at necropsy (arrow - chitosan tube).
c. Histology of intact oesophagus with its 3 normal layers and degrading chitosan tube (arrow) at day 9. (Haematoxylin eosin saffron staining, G x 2.5).
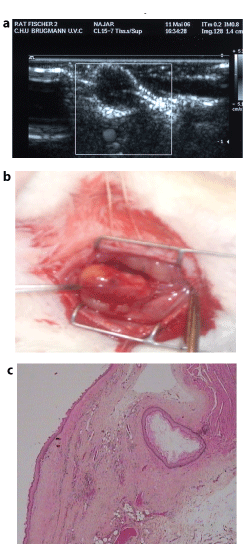
Figure 4: The grown foetal oesophagus implant.
A. Ultra sound detection and measure of the growing organoid from oesophagus-stomach graft (frame) within 2 months after implantation.
B. Histology of the organoid wall. Arrow: the cyst lumen with oesophagus epithelium surrounded by submucosa and muscle layers. (Haematoxylin eosin saffron staining. G x 2.5.))
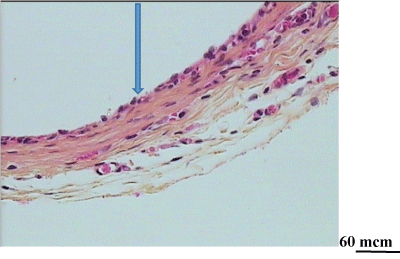
Figure 5: Degradation of a chitosan tube in the operated oesophagus. At day 7 after oesophagus repair: thin fibrous capsule covered by growing epithelium (arrow) and beginning of the muscle layer formation.No remnants of the chitosan tube. (Haematoxylin eosin saffron; G x 20).
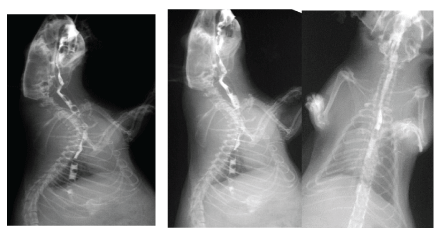
Figure 6: Video pictures of contrasted bolus transit through oesophagus within 10 months aftercircular defect repair. Arrows: limits of the surgical resection. A slight diameter decrease (less than 10%) is noted at the moment of the segment peristaltic contraction.
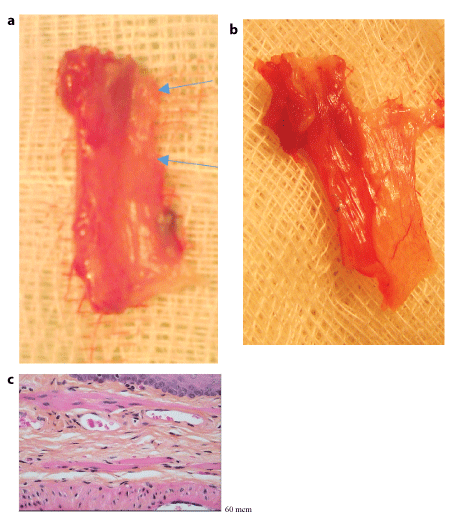
Figure 7:
(A) Macro and microscopic
(B) pictures of the cervical oesophagus within 10 months after circular defect repair.
A: Difficulties to distinguish new formed part of the organ macroscopically.
a. Whole organ,
b.longitudinally open oesophagus
Arrows: presumed limits of the new formed segment and. link with surrounding tissue;
(C) Microscopic continuity of the three layers of the normal oesophagus wall in the repaired segment. (Haematoxylin eosin saffron staining; G : x 20)
Discussion
The imaging techniques used in this work may seem primitive but they appeared adequate to solve the questions asked, i.e. morphological and functional characteristic of the cervical oesophagus at different moments after its surgical repair in a small laboratory animal (rat). The obtained data about chitosan tube degradation delays were different depending on the medium pH: 7.0-7.4 in the organism tissues, 6and less in the oesophagus lumen. That may correspond to the recent literature data which record the influence of micro environment and acetylation degree of the chitosan on its degradability [27]. The obtained by imaging information were useful for the elaboration of a specific follow up of the operated Video contrast and serial imaging of the swallow and transit of a Gastrografin® bolus has allowed the necessary evaluation of the functional and morphological results of the operation. The fact that they were controlled by and corresponded exactly to the macroscopic measures on autopsy material and to microscopic findings confirms their liability. Indeed a cinematographic investigation might be more interesting but more difficult to realize. There are few literature data about functional investigations of the oesophagus after surgical repair or regenerative treatment in rats; they are mentioned rather on porcine models of oesophagus reconstruction on artificial scaffolds [29]. Anyway thanks to the described imaging control we should consider our approach as a valuable step towards new solutions in oesophagus defect surgical repair.
Conclusion
- Roentgen and ultra sound methods of investigations using clinical devices can be applied in small laboratory animal investigations of digestive organ morphology and function.
- Roentgen video contrast and serial imaging investigation has allowed valuable qualitative and quantitative evaluation of the function and morphology of the oesophagus after severe defect surgical repair.
- The obtained in vivo imaging data has also allowed elaborating correct operation and follow up methods in oesophagus repair by combined prosthesis in small animals as a necessary step to possible human clinical applications
Acknowledgment
The authors express their thankfulness to: L.Divano and M.Canie respectively previous head and present head of the Medical Imaging Department of the CHU Brugmann for their interest and support, Mrs R Foutrel and E Marbaix(same department) for technical help, to Dr N.Damry (Medical Imaging of CHU Brugmann) ,Ms Leroy M (IPG, Gosselies), M. A.Bekkouri (ULB Laboratory of Translational Medicine)and M. Kempeneers JL for their support and logistic help.
Download Provisional PDF Here
Article Information
Article Type: CASE REPORT
Citation: Najar ES, Vandaele S, Delrée P, De Prez C, Coulic V (2018) Imaging the Esophagus Oval and Circular Defect Reconstruction in Rats. J Clin Case Stu 3(2): dx.doi.org/10.16966/2471-4925.166
Copyright: © 2018 © 2018 Coulic V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 18 Jan, 2018
Accepted date: 06 Mar, 2018
Published date: 12 Mar, 2018








