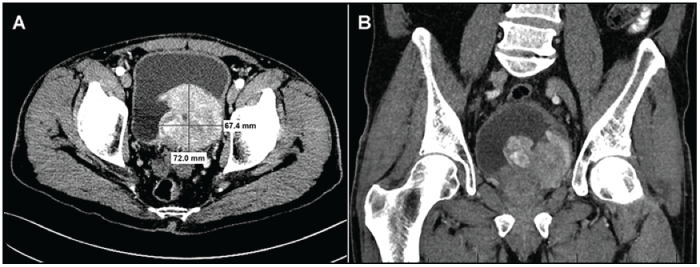
Figure 1: Computer Tomography of the urinary bladder and tumor at time of first diagnosis and before transurethral resection: (A) axial section; (B) frontal section.


Tamara P Lhungay1 Alexandra Colvin1* Jason Warncke2 Hilary Somerset3 Shandra Wilson2
1School of Medicine, Anschutz Medical Campus, University of Colorado, Aurora, Colorado, USA*Corresponding author: Alexandra Colvin, School of Medicine, Anschutz Medical Campus, University of Colorado, Aurora, Colorado, USA, E-mail: Alexandra.Colvin@ucdenver.edu
Solitary fibrous tumors (SFT) are rare, usually benign, neoplasms of mesenchymal origin. From just 16 cases reported in the urinary bladder, only two were malignant. We present here an additional case of a 70-year old man with malignant SFT of the urinary bladder with review of the current literature and discussion of the clinical pathology challenges for the diagnosis of these rare tumors. The patient had a family history of bladder cancer, and presented with dysuria, hematuria, and a 3-year history of worsening urinary incontinence. A computed tomography showed a large bladder mass with evidence of invasion into the bladder wall. This was followed by a transurethral resection of the tumor, and histopathological examination revealed a malignant SFT. After a radical cystoprostatectomy was performed, the patient is currently more than one year post-surgery without evidence of recurrence. SFT of the bladder lack distinctive patterns in symptoms, radiological imaging, and patient demographics, which can make the original clinical diagnosis challenging upon presentation. Histopathological examination may also be confused with several benign “spindle cell reactions,” and identification of malignant features including special immunostaining for tissue markers is required.
Solitary fibrous tumor; Urinary bladder cancer; Malignant fibrous histiocytoma; Urothelial carcinoma
Solitary fibrous tumors (SFT) are rare tumors that are most commonly found in the lungs, respiratory tract and mediastinum [1]. Urinary bladder occurrences of SFTs are very rare. A comprehensive review of the literature reveals 16 reported cases, of which only two were malignant [2- 15]. The average age at presentation was 54 years old. SFTs are commonly discovered incidentally, but the most common reported symptoms included pain or pressure, hematuria, and urinary discomfort (dysuria, retention, or frequency) [2-15]. When SFTs occur in an extrapleural site, they are considered benign in 78-88% of the cases [16]. Patient outcomes improve with prompt treatment, which includes complete surgical excision of the tumor with careful follow up [8]. All of the reported cases had positive survival outcomes, which makes complete surgical removal the recommended treatment for SFT of the urinary bladder [2-15]. In this study we present the third case of a malignant SFT of the urinary bladder, and summarize the current literature on SFTs of the bladder.
A 70-year-old man presented at our institution for a second opinion regarding diagnosis of a urinary bladder mass. The patient was originally seen one-month prior at an outside clinic for left flank abdominal pain, dysuria, gross hematuria and intermittent episodes of diarrhea and constipation with small stools, as well as a 3-year history of worsening urinary incontinence. An outside chest/abdominal/pelvic computed tomography with and without contrast displayed a large, 9 × 8 × 8 cm, heterogeneous enhancing mass that encompassed most of the left half of the bladder and appeared to be invading through the bladder wall with possible prostatic invasion (Figure 1). There was bilateral, mild ureterectasis but no definitive hydronephrosis. No evidence of regional adenopathy or other suspicious distant lesion was observed. The differential diagnosis included primarily an urothelial carcinoma versus other malignancies such as lymphoma, sarcoma, metastatic cancer, or benign pathologies such as blood clots and bladder stone(s). Significant laboratory findings included an elevated PSA of 5.6 ng/mL and low hemoglobin of 8.2 gr/ dL. He had a 20-pack-year smoking history and family history of bladder cancer in his father. The patient had been taking a homeopathic colloidal silver medication for two years, used to fight dysuria, and his partial thromboplastin time was consistently elevated (42 to 60 seconds). A cystoscopic examination at the outside facility confirmed the presence of a bladder mass and pathological examination of the partial transurethral resection of the bladder tumor (TURBT) was diagnosed as “solitary fibrous tumor.” After this diagnosis, the patient started using strong neodymium magnets that he would place on his abdomen, which he believed helped to alleviate some of his symptoms and prevent cancer progression.

Figure 1: Computer Tomography of the urinary bladder and tumor at time of first diagnosis and before transurethral resection: (A) axial section; (B) frontal section.
When the patient was admitted to our facility, physical examination showed a patient with a body mass index of 26.1, blood pressure of 126/70 mm Hg, pulse of 72 bpm, temporal artery temperature of 36.6°C, and a respiratory rate of 16 breaths per minute. On digital rectal exam, an extrinsic palpable mass was detected pressuring the left side, which was interpreted as an external compression of the rectum by an enlarged bladder. A second opinion pathology examination of the original specimen from the outside facility confirmed the presence of a solitary fibrous tumor but with the addition of malignant features. Two months after the first TURBT, the patient underwent a cystoscopy showing that the entire bladder was taken over by a very large tumor that was approximately 9 cm maximal dimension and arising from the left bladder wall (Figure 2). He refused resection of the tumor, thus he underwent a debulking procedure in which the tumor was resected layer by layer, noticing that this was quite vascular and it was followed by fulguration, rather than resection, with vaporization to the base. The right ureteral orifice was visualized, but the left was in the middle of the tumor resection and it could not be identified, consequently it was not possible to place a stent. The tumor was not completely resected given its size and the patient’s known coagulopathy. The tumor fragments that had been resected were sent to our pathology laboratory.
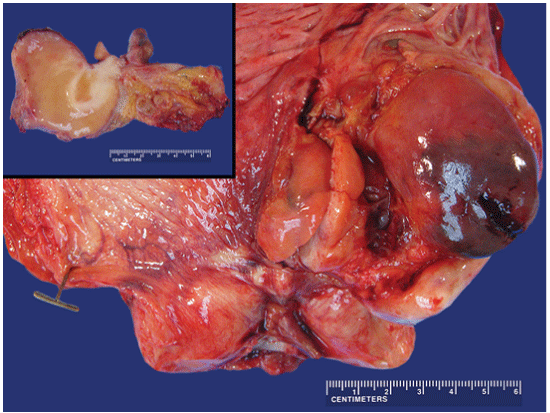
Figure 2: Gross pathology of the bladder tumor: An anterior Y-shaped incision of the urinary bladder was performed revealing an 8.0 × 7.0 × 7.0 cm multilobated mass present on the left lateral wall/trigone area of the bladder. The left upper inset shows a cross section of the tumor showing a soft tan parenchyma with focal lighter areas of firm and more fibrous tissue arising from the mucosal surface.
Pathology examination of this second TURBT revealed a spindle cell neoplasm (Figure 3) with histological features and immune phenotype most consistent with a malignant SFT. The tumor cells were positive for vimentin, CD34, CD99, Bcl-2, and were negative for pan cytokeratin (AE1/AE3), high molecular weight cytokeratin (CK 5/6), CD117 (c-kit), p63, S100, and muscle-specific actin. In several foci, the tumor was densely cellular with increased mitotic activity with up to 8 mitotic figures per 10 high power fields. Based on these features the pathology diagnosis was of SFT histologically malignant. This diagnosis was corroborated by second opinion consultation with two other in-house pathologists with a consensus diagnosis of a malignant SFT invasive into the muscularis propria, and a pathology stage pT2.
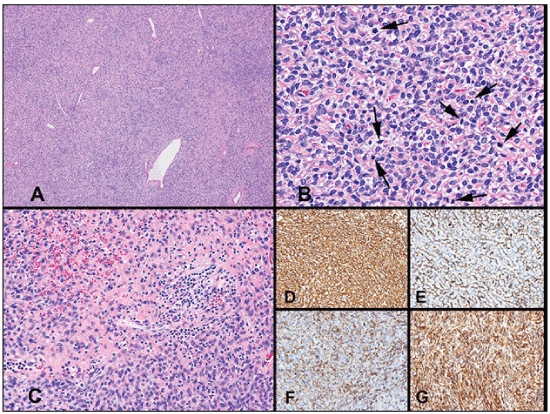
Figure 3: Histologic images of the spindle cell neoplasm: (A) Solitary fibrous tumor (SFT) with dilated, stag horn vessels (H&E, 4x). (B) SFT with increased cellularity with rounded and spindle tumor cells and increased number of mitotic figures (arrows) – features associated with malignant behavior (H&E, 60x objective). (C) Area of the tumor with focal necrosis and hemorrhage (H&E, 20x objective); Immunoperoxidase stainings (at 40x objective) for. (D) Vimentin; (E) CD34; (F) Bcl-2, and (G) CD99. Staining for CD117 (C-kit) exhibited no staining (not shown).
The University of Colorado Second Opinion Genitourinary Cancer Conference, which is comprised of urologists, medical oncologists, radiation oncologists, and pathologists, all discussed the case and recommended its best management. Since the tumor location was not amenable to partial cystectomy, the recommendation was of a radical cystoprostatectomy. However, the patient again refused this radical approach and a second TURBT was performed with pathology diagnosis identical to the previous resection. The suggestion, again, was made to pursue radical surgery, but the patient chose to attempt additional homeopathic colloidal silver therapy. After a period of over one month using this alternative therapy, and with an additional consultation with the medical oncology service, in which neoadjuvant chemotherapy was not recommended due to complete resection of the tumor as being the most prognostic of positive outcomes, as well as the lack of standard chemotherapeutic indications [3,7,15]. The patient ultimately decided to undergo a radical surgical approach.
At the time of cystectomy, the tumor was found to be significantly larger since his last cystoscopy, extending throughout the bladder from the prostate to the urachus. It measured 9 cm across the pelvis, with extension into the prostatic urethra. The surgery included radical cystoprostatectomy with extended bilateral pelvic lymph node dissection, and creation of continent Vescica Ileale Padovana (VIP) pouch with a side-to-side ileo-enteral anastomosis. The procedure length was 7 hours with 700 mL of estimated blood loss. The patient was transferred to the recovery room in stable condition and was discharged nine days post op after an uneventful post-operative hospital course.
The final pathologic diagnosis was confirmed to be a malignant solitary fibrous tumor of the bladder with a pathological staging of pT2b. There was also a prostatic adenocarcinoma, Gleason grade 3+4 that was staged pT3a with extraprostatic extension or microscopic invasion of bladder neck. In addition to the tumor samples, specimens were obtained from the left distal ureter, right distal ureter, and urethral margin, as well as from the lymph node dissections from the left obturator lymph nodes, left common iliac lymph nodes, left iliac lymph nodes, pre-sacral lymph nodes, right obturator lymph nodes, right common iliac lymph nodes, and right external iliac lymph nodes. Histological examination of these specimens showed no evidence of malignant involvement.
Adjuvant chemotherapy and radiotherapy were not administered due to the tumor’s negative margins. The efficacy of adjuvant therapy in SFT management remains controversial but is generally reserved for nonresectable, metastatic, and recurrent disease [3,7,15]. Six weeks after the operation, his hemoglobin had risen to 9.9 g/dL and his PSA was <0.01 ng/mL. Upon more than one year of follow up, the patient is well and without recurrent, residual, or metastatic disease according to MR and CT imaging taken at various times after surgery.
Solitary fibrous tumors (SFT) are rare tumors that were first described by Klemperer and Rabin in 1931 and were identified as fibrous mesotheliomas because they are commonly found in the lungs, respiratory tract and mediastinum [1]. However, other less frequent locations (e.g. salivary glands, orbit, liver, etc.) were later been described and consequently renamed as SFT [2]. In 1942 Stout and Murray learned they were derived from mesenchymal cells [17] and with the advent of immunohistochemistry, their fibroblastic origin was confirmed. In extrapleural sites, SFT are considered benign in 78-88% of the cases and will metastasize or recur in10-20% of cases [3,5]. As stated, urinary bladder occurrences of SFT are very rare; a comprehensive review of the literature reveals only 16 cases (Table 1), from which only 2 were malignant [6,7].
| Case | Age/Sex | Presentation | Size (cm) | Operation | O-NED (months) | Source | Country |
| 1 | 56/M | Frequency and sensation of residual urine | 12 × 8 × 6 | Partial Cystectomy | 12 | Kim SH et al. [12] | South Korea |
| 2 | 50/M | Pelvic pain, weight gain, and hypoglycemia | 6.5 | Radical Cystectomy | 18 | Corti B et al. [14] | Italy |
| 3 | 67/M | Incidental cystoscopy finding during TURP | 4 | Radical Cystectomy | 9 | Westra WH et al. [4] | USA |
| 4 | 67/ M | Incidental MRI finding for prostate cancer surveillance |
Unknown | TURBT | 1 | Westra WH et al. [4] | USA |
| 5 | 50/F | Incidental US finding during right hemicolectomy |
5.2 × 4.4 × 4.3 | TURBT | 18 | Bainbridge TC et al. [13] | Canada |
| 6 | 42/M | Sensation of pelvic pressure | 17 × 13.5 × 15.5 | Partial Cystectomy | 6 | Bainbridge TC et al. [13] | Canada |
| 7 | 59/M | Incomplete emptying sensation and frequency |
18 | Partial Cystectomy | 24 | Martin LL et al. [15] | Spain |
| 8 | 78/M | Hematuria and acute urinary retention | <10 cm | TURBT (2x) | 36 | Otta RJ et al. [3] | Canada |
| 9 | 54/M | Tender RLQ mass, year of recurrent pain and hematuria | 13.9 × 12.2 × 11.1 | Partial Cystectomy | 144 | Mozafarpour S et al. [11] | Iran |
| 10 | 50/M | Residual urine sensation and urethral pain | 7 | Radical Cystectomy | 9 | Wang T et al. [5] | China |
| 11 | 41/M | Abdominal bloating and weight loss | 28 × 21 | Partial Cystectomy | 8 | Dozier J et al. [6] | USA |
| 12 | 67/M | Lower abdominal pain | 16 × 9 × 9 | Partial Cystectomy | 18 | Cheng SH et al. [7] | China |
| 13 | 60/M | Incidental MRI for prostate adenocarcinoma |
3 | Partial Cystectomy | 11 | Leite K et al. [2] | Brazil |
| 14 | 23/M | Incidental cystoscopically finding, painless hematuria | 6 × 5 × 2 | TURBT | Not Provided | Wang X et al. [9] | China |
| 15 | 24/F | Hematuria and lower abdominal pain | 8.5 × 7.8 | TURBT | 24 | Heinzelbecker J et al. [10] | Germany |
| 16 | 59/F | Hematuria | 8.5 × 6.5 × 4.5 | Radical Cystectomy | 77 | Tzelepi V et al. [8] | Greece |
| 17 | 70/M | Dysuria, pain, and small stools | 8.0 × 7.0 × 7.0 | TURBT (3x) Radical Cystectomy |
12 | Current Case | USA |
Table 1: Summary of Literature Review Case Results (O-NED = Outcome with no Evidence of Disease).
SFTs are usually well-circumscribed tumors with a gray-white cut surface. Microscopic examination reveals a neoplasm with a variety of growth configurations, classically described as a pattern less or storiform arrange of spindle cells with bland nuclear morphology intermixed with dense collagen [10]. The background stroma demonstrates variably prominent hyalinization with a dilated, ramifying vascular network (so called staghorn vessels). Immunostains are characteristically positive for vimentin, CD34, CD99 and bcl-2. However, this tumor antigenic profile is very similar to that observed in gastrointestinal stromal tumors (GIST), but in our case, additional staining for CD117 (c-kit) was negative, ruling out that possibility. Interestingly, a recurrent NAB2-STAT6 gene fusion has recently been described in SFTs, and it is now being recognized that aberrations in NAB2 and STAT6 are involved in the majority of SFTs (both benign and malignant). Correspondingly, STAT6 immunostains are also positive in SFTs [6,18]. Distinction of a malignant SFT is based on their larger size (>10 cm) and the presence of increased cellularity, cellular pleomorphism, increased mitotic rate (>4 per 10 HPF), hemorrhage and/ or necrosis [7] as were also present in this case.
The prompt treatment of an SFT in the urinary bladder improves patient outcomes. The treatment currently indicated is a complete surgical excision of the tumor with careful follow up [8]. There are no standard recommendations for chemotherapy or radiotherapy in the treatment of SFTs, and the literature remains inconclusive around the efficacy of adjuvant therapy [3,7,15]. When a patient presents with a bladder tumor of mesenchymal origin, SFT should be considered in the differential diagnosis and careful histopathologic examination must be performed to rule out the presence of the above described malignant features.
A literature review was conducted using the search the terms “spindle cell lesions of the bladder,” “malignant spindle cell lesions of the bladder,” “solitary fibrous bladder tumor of the bladder,” and “malignant solitary fibrous bladder tumor.” The findings are listed in table 1. Including our case study, there are 17 reported cases of SFT of the urinary bladder from which 3 of these patients were females. The average age upon presentation was 54 years old [3,4]. Pain/pressure was the most commonly described symptom, which was reported in 7 of the patients (41.2%). Hematuria and urinary discomfort (dysuria, retention, or frequency) each were reported in 5 cases (29.4%). Notably, in 5 of the patients (29.4%), SFTs were discovered incidentally via cystoscopy (2), MRI (2), or ultrasound (1). These general and nonspecific symptoms make it difficult to differentiate SFT of the urinary bladder from other more common tumors.
Seven patients (41.2%) underwent a partial cystectomy, making it the most common treatment approach. Five patients (29.4%) chose radical cystectomy, and another five patients (29.4%) underwent at least one TURBT as their primary treatment. All these cases had positive survival outcomes, supporting the prompt, complete surgical removal of the SFT as the most recommended treatment at this time.
The average length of reported follow up was 26.2 months. One patient did not have complete follow up information. The remaining 16 patients were without evidence of disease upon last recorded follow up, with the longest being 12 years [11]. These positive outcomes illustrate the importance of complete resection of SFTs. TURBT would be a lessinvasive and complicated approach than radical cystectomy, and could be considered as the first line of treatment, mainly if invasion of the detrusor muscle is not present. Though the majority of SFTs are benign [12], upon discovery immediate TURBT should be pursued to ensure positive outcomes and minimize the chance of malignant transformation or metastasis (10-20%) [3]. This aligns with the previous discussions that have concluded that resectability is the most important prognostic factor (followed by location, margins, size, and histopathological appearance) [3]. After the resection of a SFT of the bladder, one should ensure careful follow up and surveillance.
Regarding the use of colloidal silver, its prolonged use has been found to cause coagulopathy, as evidenced in our case by a prolonged partial thromboplastin time (42 to 60 sec.) [19]. The homeopathic industry recommends its use for different purposes, from killing different pathogens to promotion of tissue healing, including to prevent urinary tract infections [20]. Even though the silver ion (Ag+) is bioactive in sufficient concentrations to kill bacteria in vitro, there is little evidence to support its medical use, and it is not recognized as safe or effective by the United States Food and Drug Administration [21]. A recent study also suggests that colloidal silver has a dose-dependent cytotoxic effect on MCF-7 breast cancer cells [22,23].
Download Provisional PDF Here
Article Type: Case Report
Citation: Lhungay TP, Colvin A, Warncke J, Somerset H, Wilson S (2017) Malignant Solitary Fibrous Tumor of the Urinary Bladder: Clinical and Pathological Challenges of a Rare Tumor. J Clin Case Stu 2(1): doi http://dx.doi.org/10.16966/2471-4925.137
Copyright: © 2017 Lhungay TP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access