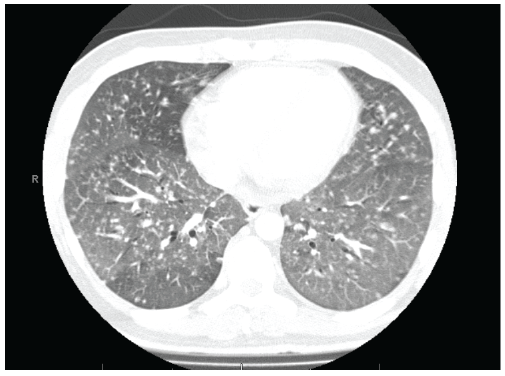
Figure 1: Chest tomography demonstrating ground glass opacities, small centrilobular nodules, and randomly distributed nodules.

Jennifer Townsend * Tobias Pusch2
1Johns Hopkins Bayview Medical Center, Baltimore, MD, USA*Corresponding author: Jennifer Townsend, Johns Hopkins Bayview Medical Center, Baltimore, MD, USA, Tel: 410-404-4732; E-mail: holmseyj@gmail.com
A 39-year old Mexican male with no past medical history presented to a teaching hospital in Dallas, Texas with dyspnea on exertion, cough, and fever. Clinical exam revealed crackles and wheezing in both lung bases. Laboratory studies revealed a positive rapid test for human immunodeficiency virus (HIV), but negative sputum cultures. His chest radiograph showed right paratracheal opacity and interstitial infiltrates. He developed worsening respiratory distress and required intubation, which was complicated by bilateral pneumothoraces and eventual death. He was diagnosed quickly with Pneumocystis jirovecii, but it took 13 days to grow Coccidioides immitis from his blood, and 22 days to grow Mycobacterium avium from his sputum, which was too late to alter his course. Given the frequency with which HIV patients acquire multiple opportunistic infections, rapid diagnostics are necessary in improving outcomes in these patients.
In the era of highly effective antiretroviral therapy, fewer patients with human immunodeficiency virus are dying of opportunistic infections (OIs). However, patients who go undiagnosed until late in the disease course may present with multiple OI’s at once, creating diagnostic and therapeutic challenges. This report describes a patient who presented with two classic OIs in addition to disseminated cocciciodomycosis. His case took nearly 2 weeks to diagnose and had a fatal outcome. Rapid diagnostics are important and can be life-saving to initiate effective therapy, and may have been useful in this case. Mechanisms of immune suppression and interaction between OIs are discussed.
The patient is a 39 year-old male from the northwestern Mexican state of Sonora, with no known past medical history who presented to the emergency room complaining of increasing dyspnea on exertion and cough for three weeks. He was active and feeling well around one month prior when he started getting progressively weak and short of breath to the point that he was no longer able to perform his job as a construction worker. Over the preceding two weeks he had developed high fevers and drenching sweats at home. He denied any rash, diarrhea, abdominal pain, or weight loss. He denied any sick contacts prior to his illness, though he reported multiple male and female sexual partners over the past year. He had been a resident of Dallas, Texas for three years and had not returned to Mexico since that time.
In the emergency department, he was noted to be febrile to 38.6°Celsius, hypoxic to 88% on room air, and tachypneic. His exam revealed a welldeveloped male in moderate respiratory distress with wheezing and crackles in the bases of both lungs. His labs were unremarkable apart from a positive rapid HIV screen. His chest radiograph showed right paratracheal opacity and interstitial infiltrates. He was admitted for evaluation, started on ceftriaxone and azithromycin, and the Infectious Diseases service was consulted. He was initiated on intravenous trimethoprimsulfamethoxazole and solumedrol for suspected Pneumocystis jirovecii pneumonia. Initial evaluation revealed a CD4 count of 18 cells/µL (365- 1437), a lactate dehydrogenase (LDH) level of 447, a (1-3)-β-D-Glucan (BDG) level >500 pg/ml (normal <60 pg/mL), and a ferritin of 9,074 ng/mL (normal <150 ng/mL). Initial bacterial sputum cultures were negative, and two sputum specimens for Pneumocystis jirovecii were positive (given below). He continued to spike fever to 39°C daily despite antibiotic therapy, and his oxygen requirements increased. On hospital day (HD) #5, a chest computed tomography (CT) was performed which revealed mosaic pulmonary attenuation, innumerable pulmonary nodules with both a random and centrilobular distribution (Figure 1), as well as enlarged mediastinal and paratracheal lymph nodes up to two cm. On HD#6 he was transferred to the intensive care unit (ICU) on 100% oxygen and was started on broad spectrum antibacterial therapy, antituberculous therapy (RIPE), and antifungal therapy with liposomal amphotericin B at 5 mg/kg IV q 24 hours.

Figure 1: Chest tomography demonstrating ground glass opacities, small centrilobular nodules, and randomly distributed nodules.
Initially his fever and hypoxia improved, but by HD#12 he suffered respiratory distress and progressive sepsis requiring intubation with paralytics, followed by shock requiring vasopressors. Over the next several days in the ICU he developed recurrent fever as well as a rash characterized by scaly plaques on his bilateral buttocks, medial thighs, and scattered papules over his chest and arms. He was noted to have thickened toenails with a blackened area of paronychia on the right foot with subungual debris, which was sent for culture. The patient unfortunately developed sequential bilateral pneumothoraces on the ventilator and refractory respiratory acidosis despite chest tube placement and maximal ventilator support. He expired on HD #27.
An extensive microbiologic workup was undertaken during his hospital stay. Routine bacterial blood cultures were submitted on admission and again on HD# 9. The latter were reported as positive for yeast after 4 days of growth. The interpretation of the gram stain was modified to fungus after further examination. These were posthumously identified as Coccidiodes immitis/ posadasii. Biopsies were taken of the left arm which revealed endovascular spherules and endospores (Figure 2). Eventually, Coccidioides was isolated from multiple blood and fungal cultures including blood cultures from HD#4, 12, 13,16, 20, and 24; sputum cultures, tissue culture from his left arm, a skin swab of his groin, and clippings from his toenail. A gram stain of the blood culture bottle is shown in Figure 3.

Figure 2: Skin biopsy showing endospherules of Coccidioides in the dermis. H and E,40x

Figure 3: Gram stain of a blood culture bottle showing hyphae of Coccidioides immitis/posadasii. 100x, oil immersion.
A blood interferon assay for tuberculosis (T-spotTM) was negative. Four sputum specimens were submitted for AFB smears which were negative. On HD#22, two of his AFB sputum cultures turned positive, which were posthumously identified as Mycobacterium avium-intracellulare (MAI).
Sputum specimens were submitted and decontaminated for mycobacterial identification per CDC protocol [1]. The resulting sediment was inoculated onto two Löwenstein-Jensen (Gruft modification) and one Middlebrook 7H10 slants (both Remel™; Lenexa, Kansas, USA) and incubated at 37°C in ambient air. An aliquot was pipetted into a mycobacterium growth indicator tube (MGIT™ Becton Dickinson BBL™, Sparks, Maryland, USA) containing modified 7H9 broth base, OADC growth supplement and 0.8 ml of reconstituted, lyophilized PANTA. The tube was incubated in 37°C in ambient air in an automated BD Bactec MGIT Instrument. The instrument identified the tube as positive on HD #22 which was confirmed by Ziehl-Neelsen staining and again subcultured to slants as stated above. The acid-fast positive MGIT broth or solid culture colonies respectively were then tested using nucleic acid hybridization probes for M. tuberculosis and M. avium complex, utilizing the Leader 450i luminometer system (Gen-Probe Inc., San Diego, CA, USA), irrespective of colony morphology due to the high incidence of both mycobacteria at Parkland Memorial Hospital.
The isolate was identified as M. avium complex after generating a relative light unit (RLU) value greater than the pre-specified cut-off. No phenotypic identifications were performed.
Respiratory specimens of induced sputum were transferred to a sterile container and sent out to Parkland’s reference laboratory, ARUP (Associate Regional and University Pathologists; Salt Lake City, Utah), for Pneumocystis Direct Fluorescent Antibody Staining.
Blood for fungal cultures were collected in Isolator tubes (Wampole Isolator 10TM Microbial Tubes, Cranbury, NJ, USA), and processed according to manufacturer recommendations under the biosafety cabinet. Blood and skin tissue culture specimens were inoculated onto potato dextrose agar (PDA), Brain-Heart infusion (BHI) agar with blood and Sabhi slants (all Remel™; Lenexa, Kansas, USA). The PDA and Sabhi slants were incubated at room temperature. The BHI with blood slant was incubated at 37°C with untightened cap to allow oxygenation. Nonsterile specimens (sputum and toenail) were inoculated onto Sabourauddextrose with chloramphenicol/gentamicin agar, Inhibitory Mould with gentamicin agar (IMA) and a BHI with blood/chloramphenicol agar plate (all Remel™; Lenexa, Kansas, USA). The former were incubated at room temperature in ambient air, the latter at 37°C. All culture plates were shrink-sealed.
The cultures were examined weekly for growth for six weeks. Upon noting colony growth of gray, moist colonies, a lacto-phenol cotton blue tape preparation was performed. If no conidia are seen and no mould identification is possible, a colony is sub-cultured from the primary slant onto a PDA agar plate and slide cultures are prepared. In the case of this patient, the identification of Coccidioides species was made based on microscopic and colony morphology. This was resulted as ‘Coccidioides immitis/posadasii’ as no differentiation can be made between the two species on phenotypic basis alone.
To our knowledge this is the first report of a triple coinfection with Coccidioides immitis/posadasii, MAI, and Pneumocystis jirovecii pneumonia (PJP). The patient had clinical and radiographic evidence of all three pathogens. He had ground glass opacities, pneumothoraces, and a partial response to trimethoprim-sulfamethoxazole, which may represent features of Pneumocystis [2,3]. His clinical syndrome also met criteria for nontuberculous mycobacterium infection, including a nodular infiltrate, mediastinal adenopathy, and two positive sputum cultures for Mycobacterium avium intracellulare [4]. However, Coccidioides was the dominant and disseminated pathogen which most likely led to his demise.
With improvements in diagnosis, the frequency of polymicrobial infections in HIV patients is increasing. Previous cases reported in immunocompromised patients have noted dual infections of Pneumocystis with either fungi or mycobacterium, but not all three together. Pneumocystis has been seen as a frequent co-pathogen with other fungi including Cryptococcus, Histoplasmosis and Aspergillus, but not with Coccidioides as of this report [3,5-11].
Pneumocystis has also been reported as an agent of coinfection or colonization in patients with Mycobacterium tuberculosis [12], and also rarely with Mycobacterium avium intracellulare in one unusual case causing a coinfection of the choroid [13]. Given the near complete overlap in risk factors for PJP and MAI, coinfection is theoretically expected to be frequent. Coccidioides and Mycobacterium tuberculosis (MTB) share numerous risk factors and are frequently seen together, especially with HIV infection and imprisonment in endemic areas [14], however Coccidiodes and MAI coinfections have not been reported.
Mycobacterium avium complex (MAC) is composed of two closely related species that are difficult to differentiate, Mycobacterium avium, and Mycobacerium intracellulare, denoted together as Mycobacterium avium-intracellulare (MAI). MAC is composed of several serovars and strains of variable pathogenicity in humans [15]. Differentiation is based on serovar-specific glycopeptolipids (ssGPLs), which are thought to contribute to virulence [16]. Mycobacterium avium serovars 1, 2, 4 and 8 are responsible for 95% of AIDS-related infections. These mycobacteria are ubiquitous soil derived organisms distributed worldwide, and may impact up to 20-40% of HIV patients with a CD4 <50 in the absence of prophylaxis [17]. MAC tends to flourish in areas where M. tuberculosis has been controlled [18]. In AIDS, MAC presents as a disseminated infection, especially the lungs (cavities and nodules), gastrointestinal tract (GI), bone marrow, and lymph nodes [19-24]. Diagnosis of MAC is made by specimen culture in solid or liquid media as described above, followed by biochemical identification or molecular methods. Disseminated MAC is diagnosed by a positive culture from blood or bone marrow culture with the yield of each around 30% [25]. Isolation from blood or bone marrow requires the use of acid-fast bacilli-isolator blood-culture tubes with lysiscentrifugation and subsequent culture [18].
DNA probes for MAC targeting the mycobacterial ribosomal 16S RNA have become available for clinical use in the last decade and allow the identification of MAC from a positive culture in a few hours. Rapid culture methods (BACTEC[TM] MGIT[TM] and MB/bacT), in combination with DNA probes accelerates the diagnosis of mycobacterial disease (Accu Probe) [26]. PCR amplification directly from specimens may further accelerate diagnosis, but is not widely available [27]. Matrixassisted Laser Desorption Ionization-Time of Flight Mass spectrometry (MALDI-TOF MS) is increasingly used for NTM identification from culture, but not from clinical isolates [28]. Treatment for proven disease includes clarithromycin or azithromycin in addition to ethambutol, continued for at least 12 months if macrolide sensitivity is confirmed. In cases of high bacterial burden, absence of HAART, or CD4<50, rifabutin is recommended as a third agent [17].
Coccidioides immitis and posadasii are the two species in the genus Coccidiodes and are the causal agents of “Valley Fever”. They are dimorphic molds found in the soil in a narrow geographic range, including the desert regions of the American Southwest and a majority of Mexico. Infection occurs through inhalation of arthroconidia into the lungs followed by hematogenous dissemination to multiple organ systems. A majority of infections (60%) are asymptomatic. Others are self limited respiratory infections, but 5-10% result in chronic pulmonary sequelae including ongoing cough, sweats, weight loss, cavitation, and mass-like consolidation which can invade surrounding tissue planes. Rash is a common finding in extrapulmonary disease and can present in a wide variety of ways, including a papular rash in acute illness, verrucae, acne (as in our patient), ulcerated plaques, abscesses, and fistulae [29,30]. Complications in disseminated disease include miliary lung disease, meningitis, osteomyelitis, or multiorgan involvement [29,31,32]. HIV patients with a CD4 count <250/uL are at disproportionate high risk for dissemination, which occurs in 30-50% of cases. Genetic studies of patients with disseminated Coccidioides without known immune deficits have identified mutations in the IFN-γ and IL-12 receptors as predisposing factors confirming the importance of this axis in fungal control [31-35].
Timely diagnosis of Coccidioides requires a high suspicion and aggressive pursuit of specimens for tissue pathology and culture. While serologies are useful for immunocompetent persons, false negative rates are high in AIDS patients given a lack of robust humoral response [33]. Direct tests for fungal antigens including galactomannan can be useful early in infection during the endospore producing phase [33,34,35,36], but require the use of a reference lab, which can delay diagnosis for 5-7 days. The gold standard for diagnosis is histology demonstrating the pathognomonic spherules [36], which was obtained in our patient immediately upon appearance of the skin rash, with results taking 48- 72 hours. PCR of clinical specimens is highly sensitive and specific in experimental studies and clinical studies, but has not been widely validated [37,38].
Recommended treatment of serious Coccidioides infections begins with amphotericin B (vertebral osteomyelitis, rapidly progressive pneumonia) or high dose fluconazole up to 2000 mg daily (meningitis) for several weeks until improvement is seen followed by oral fluconazole for at least one year or lifelong in the case of meningitis [36]. Surgical debridement may be required for local control of cavities and abscesses. HIV patients should remain on secondary fluconazole prophylaxis until the CD4 count rises >250 cells/µL.
Pneumocystis jiroveci, the agent of Pneumocystis pneumonia (PCP), has a convoluted microbiological history. Since originally classified as a form of T. cruzi by Carlos Chagas in 1909, it was thought to be a protozoan until reclassified as a fungus by rRNA sequence homology in 1989 [39]. Studies of epidemiology, transmission, and pathophysiology have been limited by the nonexistence of a viable culture technique. Serologic studies indicate that a majority of persons are exposed at a young age, while others suggest that person to person transmission and de novo infection play a role in at risk hosts [40]. Colonization is a frequent event in a wide range of hosts including persons on chronic steroid therapy (44%), and persons with HIV (40-69%) [40] PCP presents with subacute dry cough, dyspnea, and fever. Chest radiograph findings include bilateral reticular or nodular infiltrates extending out from the hilum, ground glass opacities, parenchymal cysts, or rarely focal consolidation [40]. PCP does not usually cause adenopathy or pleural effusions. Diagnosis currently involves direct visualization of the organism in BAL fluid, induced sputum, or lung tissue using a variety of techniques (methenamine silver, Giemsa, toluidine blue, Diff-Quick, or immunoflourescence). The sensitivity of these assays is highly variable depending on organism load and lab experience, and may be as low as 50%, mandating the submission of multiple specimens or BAL for diagnosis.
BAL or lung biopsy is preferred, given the higher sensitivity of 90- 100% in these specimens [17]. Similar to other infections, PCR has demonstrated high sensitivity and specificity in PCP, up to 98% and 96% respectively when using RT-PCR at the heat shock protein 70 gene in BAL fluid [40]. A PCR assay targeting the cdc2 gene is also highly sensitive and specific and is available in reference laboratories [41].
The tendency of one infection to lead to another is an unfortunate observation that has fueled extensive immunological investigation. HIV patients commonly have polymicrobial pulmonary infections (7-14% in pneumonia, and up to 85% in cavitary disease) [42-44]. While the pathways of synergistic disease progression have been extensively studied for the most common HIV copathogens (MTB and Hepatitis C) less work has been done to uncover the interactions in polymicrobial respiratory infections.
A case is mounting for the immunomodulatory role of MAI as a gateway to other pulmonary infections. Cell mediated immunity, in particular the Th17 response, is central to the body’s defense against MAI, PJP, and Coccidioides [40,45]. In vitro studies have demonstrated that MAI manipulates the immune response so as to upregulate IL-17 expression early in infection leading to chemotaxis of abundant immune effectors (MAI infection targets) and subsequently suppress the downstream immune response. The bacterium downregulates NFκB within 1 hour of infection and controls the host’s ability to trigger phagolysosome fusion. MAI thus rescues itself from intracellular killing to persist within macrophages. MAI also triggers anti-inflammatory cytokines (IL-10 and TGF-β), which downregulate macrophage killing and may allow it to survive within fused phagolysosomes [16,45]. Moreover, MAI reduces macrophage responsiveness to IFN-γ and suppresses STAT-1 phosphorylation. These changes reduce macrophage TNF-α production, which is central to mycobacterial killing. Thus, patients with persistent MAI infections have incapacitated macrophages and a dampened IFNγ response, making them vulnerable to multiple other pulmonary pathogens normally cleared by this pathway.
In retrospect, each of the diagnoses eventually made in our patient were associated with significant delay which may have negatively impacted his outcome. The care of immunocompromised patients with polymicrobial infections would be greatly enhanced by the development of rapid multiplex assays, such as the one recently reported by Gago et al. [46] in April 2014 [16,46]. Rapid and complete microbiologic diagnosis could assist clinicians in the initiation of effective treatment and cessation of antibiotics with overlapping toxicities.
While Hickam’s dictum (“patients can have as many disease as they damn well please”) has been the ruling paradigm in HIV medicine for several decades: clinicians remain ill equipped to rapidly diagnose, prioritize, and treat numerous simultaneous pathogens. Hopefully in the future, rapid and accurate diagnostics will become available to assist in the rational and timely care of these patients.
The authors acknowledge Stacy Beal, MD for providing the microbiology slides for this case.
None
The University of Texas Southwestern Infectious Disease Division provided time for this research project.
Download Provisional PDF Here
Aritcle Type: Case Report
Citation: Townsend J, Pusch T (2016) Lethal Coinfection with Coccidiodes immitis/posadasii, MAI, and Pneumocystis jirovecii as a First Presentation of HIV. J Clin Cas Stu 1(2): doi http://dx.doi. org/10.16966/2471-4925.108
Copyright: © 2016 Townsend J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access