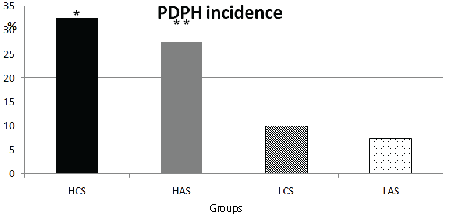
Figure 1: Comparison of the groups for PDPH incidence
*When patients with PDPH in Group HCS were compared Group LCS and Group LAS (p=0,029 and p=0,012respectively)
**When patients with PDPH in Group HAS were compared with Group LAS( p=0,039).

Zakir Arslan1* Dilek Kutanis2 Ahmet R Ozkan2 Mahmut Aslan2 Engin Erturk2
1Regional Training and Research Hospital, Department of Anesthesiology and Intensive Care, Erzurum, Turkey*Corresponding author: ZakirArslan, MD, Department of Anesthesiology and Intensive Care, Regional Training and Research Hospital, 25240, Erzurum, Turkey, Tel: + 90 442 232 55 55; Fax: + 90 442232 5025/90; E-mail: zakir-arslan@hotmail.com
Article Type: Research Article
Citation: Arslan Z, Kutanis D, Ozkan AR, Aslan M, Erturk E (2016) The Effect of Geographic Altitude on Frequency of Postdural Puncture Headache. Prospective Multicentric Study. J Clin Anesth Manag 1(5): doi http://dx.doi.org/10.16966/2470-9956.119
Copyright: © Arslan Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Background: Many risk factors have been described for (Postdural puncture headache) PDPH. No study compared incidence of PDPH at different geographic altitudes before. We think that geographic altitude may influence the incidence of PDPH.
Material and methods: This is a prospective, multicentric, observational and case-control study conducted in two different cities. One of these cities has 1890 meters altitude, the other is placed the sea side. A total of 160 patients who had been operated for caesarean section or simplistic anal surgery under spinal anesthesia were divided into four equal groups: At high altitude (H): caesarean section (Group HCS) and anal surgery (Group HAS) groups; At low altitude (L): caesarean section (Group LCS) and anal surgery (Group LAS) groups. The presence of PDPH and patient’s verbal rating scale (VRS) at the worst painful moment through the first five days were asked to all patients and were discussed.
Results: The frequency of patients who stated experiencing PDPH in Group HCS were higher than those in Group LCS (p=0. 029). The patients experiencing PDPH in Group HAS were also higher than those in Group LAS (p=0.039). The total number of PDPH in patients living at high altitude (n=24) were higher than those living at low altitude (n=7), (p=0. 001). VRS values in Group HCS were also higher than those in Group LCS. VRS values in Group HAS were also higher than those in Group LAS.
Conclusion: Geographic altitude seems to have a role in PDPH incidence and severity.
Postdural Puncture Headache, Altitude
Postdural puncture headache (PDPH) is one of the most important complications of neuroaxial blocks. It has been a well known phenomenon more than a century. There are some theories to explain PDPH. After the spinal or epidural injection, breach in the dura mater causes cerebrospinal fluid (CSF) leakage through the dural rupture [1]. Reduction of CSF volume in the subarachnoid place causes lower intracranial pressure and traction on pain sensitive structure within the cranium [2]. Additionally, arterial and venous dilatation also plays a role in PDPH [3]. The headache usually develops within the 7 days after the intervention, worsens within the 15 min of assuming the upright position, improves within 30 min of resuming the recumbent position and usually disappears within 2 weeks [1-4].
Risk factors for PDPH include female gender, young age, low body mass index and history of prior PDPH [5]. Many studies were conducted to decrease the PDPH. Some of these are related to the anesthesia technic, needle size [6-9], needle type [10-14], needle design [15], direction of bevel [16-18]. Then, general comments were introduced for the potential benefit of greater needle gauge, needles with a pencil-point design, paramedian direction and lateral decubitus positioning for preventing PDPH. As a consequence of these studies, PDPH incidence is decreased from 66% to 5-10% [19]. However, PDPH is still an important complication of spinal and epidural anesthesia.
The effects of environmental conditions on PDPH have not been sufficiently investigated so far. One of the environmental conditions that must be taken into the consideration is altitude effects on the atmospheric pressure and intracranial pressure. There are just a few case reports in the literature in this field [4,20]. We hypothesized that CSF leakage may be affected by the atmospheric pressure. The effect of atmospheric pressure on PDPH has not been investigated clinically. The aim of this study was to examine the effect of atmospheric pressure differences arising from geographic altitude on PDPH incidence.
This is a prospective, multicentric, observational and case-control study. After obtaining the ethics committee approval, the study was conducted between April, 2013 and August, 2013 in two different hospitals (Karadeniz Technical University Medical Faculty Hospital and Erzurum Private Sifa Hospital) in two different cities, Trabzon and Erzurum. Erzurum is located in eastern Turkey, approximately 1890 meters above the sea level. Trabzon is located in northern Turkey, beside the Black Sea with a mean altitude of 10 meters.
Informed written consent was obtained from 160 ASA physical status I and II patients, aged between 18-45 years (40 patients undergoing elective cesarean delivery under spinal anesthesia, 40 patients undergoing elective simplistic anal surgery under spinal anesthesia) in each city. Patients were allocated to one of four groups, consecutively: caesarean section (CS) and anal surgery (AS) groups at high altitude (H) region in Erzurum (Group HCS; n=40, Group HAS; n=40, respectively), and at low altitude (L) region in Trabzon (Group LCS; n=40, Group LAS; n=40, respectively).
PDPH is defined as a throbbing or dull headache in a frontal-occipital distribution which appears after a lumbar puncture, most occur within the first 3 days following the procedure, and improves when the patient is supine position and worsens with sitting upright position [21].
|
|
|
|
|
|
|
Inclusion criteria |
|
|
|
|
The patient’s age, ASA score, body mass index (BMI) and gender were recorded. No patients were sedated preoperatively. In the operating room electrocardiogram, noninvasive blood pressure and pulse-oximeter were monitored and recorded. All patients were administered iv 300 ml of 0.9% NaCl before spinal anesthesia. In left lateral decubitus position, spinal anesthesia was applied to all patients with the same protocol as 12.5 mg of 0.5% hyperbaric bupivacaine at L3-4 midline inter spinal space using 25-gauge atraucan spinal needle. Then the patients were turned to supine position and surgeries were performed after reaching adequate anesthesia. Intraoperatively,1000 ml of 0.9% NaCl were given to each patient. In the postoperative period, all patients were administered same analgesic protocol (500 mg of paracetamol intravenously if required) and 1000 ml of 0.9% NaCl. All patients were discharged at the end of 24 hours of followup in hospital.
Before discharge, all patients were informed about PDPH. It was described that the headache worsens within the 15 min of starting the upright position, improves within 30 min of bed rest position. If PDPH occurrence, patients were recommended hydration and bed rest treatment. If the headache does not lighten, the patients were told to come back to the hospital for further treatment. All patients were also taught about verbal rating scale (VRS; 0: no pain, 10: the worst pain, excruciating). At the end of the postoperative 5th day, all the patients were phoned by the anesthesia nurse who knew but did not involve in the study. The patients were asked and recorded whether or not the headache (PDPH) exists and verbal rating scale at the worst painful moment.
Characteristics were presented as mean and standard deviation (SD) for continuous variables and number and percentage as categorical variables. The Kolmogorov–Smirnov test was used to determine normality and homogeneity of data distribution. Continuous variables were compared using Student’s t test and Pearsons’chi square test was used for the comparison of categorical variables. A P value of less than 0.05 was considered as statistically significant. Statistical analyses were conducted using SPSS, v.18.0 (SPSS Inc., Chicago).
There were no statistical differences between the groups with respect to age, ASA score, BMI, and also gender (Table).
The number of patients who stated experiencing PDPH in Group HCS were higher than those in Group LCS (p=0. 029) (Table 1). Thirteen patients in Group HCS, 4 patients in Group LCS and 3 patients in Group LAS complained PDPH. The number of patients experiencing PDPH in Group HAS (n=11) were also higher than those in Group LAS (p=0.039) (Table 1, Figure 1). The total number of PDPH in patients living at high altitude (n=24) was higher than those living at low altitude (n=7), (p=0. 001), (Figure 2).

Figure 1: Comparison of the groups for PDPH incidence
*When patients with PDPH in Group HCS were compared Group LCS and Group LAS (p=0,029 and p=0,012respectively)
**When patients with PDPH in Group HAS were compared with Group LAS( p=0,039).
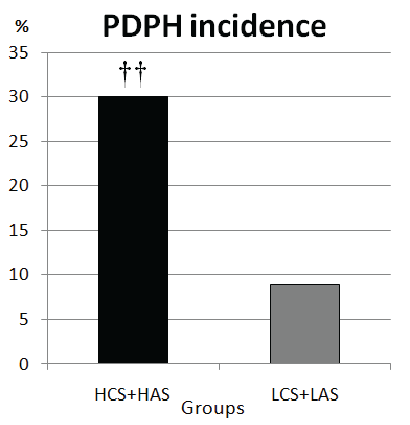
Figure 2: Comparison of the patients at high and low altitude
††: When patients with PDPH at high altitude (HCS+HAS) were compared with at low altitude (LCS+LAS) p=0.001
VRS values in Group HCS were also higher than those in Group LCS (p=0, 005). VRS values in Group HAS were also higher than those in Group LAS (p=0, 031), (Table 1).
|
Group HCS n=40 |
Group HAS n=40 |
Group LCS n=40 |
Group LAS n=40 |
Age (years) |
28.6 ± 12.3 |
36.7 ± 10.2 |
31.6 ± 9.3 |
38.4 ± 11.6 |
ASA score (I/II) |
35/5 |
30/10 |
34/6 |
29/11 |
Body mass index (kg/m2) |
23.8 ± 4.7 |
24.2 ± 3.7 |
23.1 ± 5.6 |
23.6 ± 6.0 |
Gender (M/F) |
(0/40) |
(5/35) |
(0/40) |
(8/32) |
PDPH incidence (%) |
13(32.5) * |
11(27,5) ** |
4 (10) |
3 (7,5) |
VRS |
3.2 ± 3.7† |
2.5 ± 3.3†† |
0.9 ± 1.9 |
1.4 ± 2.8 |
With this study, we showed the importance of the environment, namely the effect of geographic latitude, in the frequency PDPH. This is the first study comparing PDPH frequencies in different geographic altitudes. We found that PDPH incidence is higher at high altitude region compared to lower altitude. Moreover, the difference between the geographic altitudes is more evident in women who were operated for cesarean section. It seems that external atmospheric conditions affect headache incidence.
Atmospheric pressure is affected by external factors such as geographic altitude, temperature, wind, moisture and etc. Geographic altitude is the most important factor affecting the atmospheric pressure. In the sea level, the average atmospheric pressure is about 1013 hPa (hectopascal) and when altitude raises, atmospheric pressure decreases. The CSF pressure towards the dura mater and ligamentum flavum may relatively increase in high altitude region. As a result, we hypothesized that CSF leakage that causes headache increases in patients who live in low atmospheric pressure land. Higher incidence of PDPH in high altitude groups supports our hypothesis.
The effects of altitude on anesthesia have been discussed for many years. In 1964, Safar and Tenicela stated that postspinal anesthetic headache was so high in the high altitude land (Peru) that “it almost calls for prohibition of the use of this method” [22]. Although there is a tendency that increased PDPH is associated with high altitude land, this hypothesis has not been sufficiently supported by clinical studies before. There are only a few case reports emphasizing this association: Thirty six hours after the spinal anesthesia without complications, Panadero et al [20] reported PDPH in their patient on take-off position during air travel. The headache started 10 min after take-off and decreased after landing. Likewise Porhomayon et al. [4] reported a case of prolonged delayed PDPH exacerbated by air travel. They speculated that this rapid decompression during take-off may have altered the relationship of epidural and dural pressures. Thus, CSF leakage resulted in PDPH increase during air travel especially in take-off and landing position. As airplanes fly in altitudes with very low atmospheric pressures that are incompatible with life, they have to adjust the cabin pressure at physiological levels. This cabin pressure corresponds to a certain altitude over the world. According to existing guidelines, the maximum cabin altitude allowed is 2440 during flight [23].
The alterations of altitude may directly affect the patient’s CSF pressure and may result in headache. It was demonstrated in experimental study that CSF pressure was increased in response to hypobaric stress with simulated altitude [24]. In a clinical study, Singh et al. measured CSF pressure invasively in 34 soldiers who were transported rapidly via helicopter from sea level to high altitude region. They found that CSF pressures were increased compared to baseline levels. Consequently they concluded that CSF pressure regulatory mechanism may have been impaired with rapid altitude changes [25].
In our study, increased incidence of PDPH in high altitude groups compared to low altitude groups may be due to increase in CSF leak as a result of pressure differences between subdural space and the atmosphere. Incidence of patients who experienced headache and also the VRS scores in high altitude region were higher than those who live in low altitude. However, this difference was more apparent between caesarean groups. Epidural veins are related with intraabdominal veins. Intraabdominal pressure considerably increases in last term of the pregnancy; this pressure is reflected from the epidural area to the dura and subarachnoid space. After the caesarean section, intraabdominal and epidural pressure suddenly decreases. Then, we think that CSF pressure which is relatively increased towards dural puncture causes more leakage and higher incidence of PDPH. In patients who were operated caesarean section, this CSF leakage may be more in highland than lowland due to pressure differences.
Pain threshold affects the pain prevalence and incidence. It may vary according to culture, region, mood of person, and etc. As a twenty year experiences in regional anesthesia Turkish people’s threshold is generally low. Moreover there are differences between the pain thresholds from region to region. Erdine et al. investigated pain prevalence according to regions of Turkey [26]. They found that the pain threshold in northern Turkey (including Trabzon, close the sea side) was lower than eastern Turkey (including Erzurum, a high altitude region). In our study, it was expected, according to Erdine’s study that PDPH incidence and VRS values in Trabzon might be higher than in Erzurum. But, we found opposite findings that underlines the effect of altitude irrespective of regional differences in pain perception. Our finding, be found contrary to expectation, support increase CSF leakage leading to PDPH and presented theory.
Although these findings support our hypothesis, there are some limitations in this study. First, as we compared the patients who lived in different geographic and cultural region, pain threshold of patients differs according to their sensations, educations or culture of life. If the same patients were used in both altitudes, these results could be more credible. On the other hand, both anesthetists and surgeons in highland and lowland were not same persons. Thus, the surgery and pain evaluation may not be reflecting results correctly. Second, in our study the incidence of PDPH in both altitude lands are relatively higher than the literature. Telephone inquiry to detect PDPH may lead to upper estimate. Third, the interviewers were not blinded to patient status even if not know each other’s results. If the person who didn’t participate to the study inquired the presence of PDPH, the results might be more precious. Fourth, we just investigated the presence of headache and VRS score on the fifth day. It would be better to design the study as having VRS scores on each day until the day of seventh or tenth. Sixth, we could not measure CSF pressures in our study groups. If we were able to measured CSF pressure, we would get more reliable results.
In conclusion, we consider that, PDPH seems more frequently encountered after dural puncture at high geographic altitude region than lower geographic altitudes. Therefore, patients should be advised of an increased risk of PDPH. However, further studies are also required to define the effects of geographic altitude and atmospheric pressure on PDPH incidence.
none
none
Download Provisional pdf here
All Sci Forschen Journals are Open Access