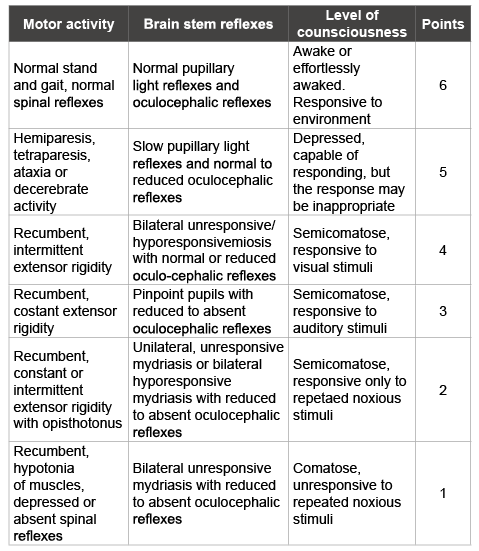
Table 1: Modified Glasgow coma scale (adapted by Platt et al. [7]) used to evaluate the neurological status after intracranial surgery.


Daniela Casoni1* Annalisa EJ Giovannini1 Christina M Precht2 Chiara Adami3
1Department of Clinical Veterinary Sciences, Anesthesia and Pain Division, University of Berne, Switzerland*Corresponding author: Daniela Casoni, Department of Clinical Veterinary Sciences, Anesthesia and Pain Division, University of Berne, Switzerland, Tel: 0041 (0)31 6312288; Fax: 0041 (0)31 6312620; E-mail: daniela.casoni@vetsuisse.unibe.ch
Article Type: Case Report
Citation: Casoni D, Giovannini AEJ, Precht CM, Adami C (2016) A Possible Case of Neurogenic Pulmonary Edema in a Sheep following Intracranial Surgery. J Clin Anesth Manag 1(3): doi http://dx.doi. org/10.16966/2470-9956.110
Copyright: © 2016 Casoni D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Objective: To describe presentation, treatment and outcome of a sheep that developed acute respiratory distress after intracranial surgery
Case summary: A 3-year-old female crossbred sheep weighing 64 kg was anaesthetized for intracranial surgery as a part of a research project. Premedication and induction of anesthesia were uneventful as well as tracheal intubation. Anesthesia was maintained with isoflurane in a 50% mixture of oxygen and air, fentanyl (5-15 µg kg-1h-1) and lidocaine (1.8 mg kg-1h-1). During anesthesia, an increased alveolar-arterial oxygen gradient was calculated on the basis of arterial blood gas analysis: inspiratory fraction of oxygen was increased and a recruitment manoeuvre was performed. After 210 minutes of anesthesia, the sheep was let recover with oxygen supplementation under monitoring of pulse-oxymetry, capnography, inspired and expired oxygen, temperature and invasive blood pressure. At tracheal extubation no signs of regurgitation or aspiration were noticed. Twenty-five minutes later, the sheep showed deterioration of neurological status and clonic seizure responsive to diazepam. After transient tachycardia, blood pressure rose acutely and sinus bradycardia followed. Severe tachypnea started in few minutes accompanied by loud respiratory noises and harsh diffuse crackles on both sides of the thorax. Foamy blood nasal exudates discharged from the nostrils. Neurogenic pulmonary edema as a sequel of increased intracranial pressure was suspected and treated with intravenous mannitol (0.5 gkg-1) and furosemide (4 mgKg-1). Hypoxemia was successfully managed with oxygen supplementation. Motor and cognitive functions improved progressively and were deemed normal within 12 hours from the episode, when arterial partial pressure of oxygen was 11.7 kPa (88 mmHg) at room air.
New information provided: Severe pulmonary complications may be observed after iatrogenic neurological injury in sheep. Neurogenic pulmonary edema should be taken into account among the differential diagnoses of abrupt respiratory distress following seizure.
Neurogenic pulmonary edema; Non-invasive blood pressure; Normoglycemia
fR: Respiratory rate; IBP: Invasive blood pressure; NIBP: Non-invasive blood pressure; ICP: Intracranial pressure; NPE: Neurogenic pulmonary edema; NPPE: Negative pressure pulmonary edema
Sheep are extensively used as animal models for intracranial surgery due to the similarity of their brain with the human one in terms of dimensions and physiology. However, very little is reported in literature concerning postoperative neurosurgical sequelae in small ruminants. Pulmonary complications are of particular interest since they are prevalent in critically ill neurological patients and potentially fatal [1].
Neurogenic pulmonary edema (NPE) is a clinical syndrome characterized by acute onset pulmonary edema following a significant central nervous system (CNS) insult. Several CNS events leading to acute increase of ICP have been associated with this syndrome in human beings. NPE has also been induced in experimental dogs [2] and hypothesized in clinical canine patients [3,4]. There is evidence of experimentally-induced neurogenic pulmonary edema in sheep [5,6]; however spontaneous NPE has never been reported in this species. The authors aimed at reporting a case of possible NPE in a sheep during recovery from general anesthesia after deep brain electrodes implantation.
A 3-year-old female cross bred sheep weighing 64 kg was anaesthetized as a part of a research project. The study received ethical approval by Cantonal Office of Bern (BE 33/13) and aimed at evaluating the functionality and biostability of brain electrodes for deep brain stimulation. The surgical procedure consisted of a craniotomy and bilateral insertion, of the stimulating device in the nucleus caudatus. The position of the electrodes was postoperatively verified with CT images, CT showed neither signs of intra- and extraaxial bleeding nor cerebraledema.
The sheep was admitted to the Clinical Veterinary Hospital the day before the trial; food was withheld for 18 hours prior to general anesthesia, but free access to the water was granted. The animal was judged healthy on the basis of anamnesis and physical exam, which was limited by its frantic behavior to manipulation. A venous blood gas analysis revealed normocapnia, normoglycemia and normal serum electrolytes concentrations. The day of the trial, sedation was provided with a combination of intramuscular midazolama (0.5 mg kg-1) methadone (0.2 mg kg-1) and medetomidinec (10 µg kg-1). Thereafter, a single lumen Seldinger catheterd was placed in a jugular vein and induction of general anesthesia was achieved with intravenous lidocainee (2 mg kg-1) and propofolf to effect, aiming at the suppression of gag reflex (total dose 2.5 mg kg-1). Induction of general anesthesia and tracheal intubation were smooth and uneventful. After tracheal intubation, balanced anesthesia was maintained with isofluraneg at Et of 1.5-1.8% (1-1.2 MAC) in a mixture of oxygen (50%) and air (50%) plus intravenous fentanylh (5-15 µg kg-1h-1) and lidocainee (1.8 mg kg-1h-1). Plasma Lytei solution was infused at 5 mL kg-1h-1 throughout the procedure. Mechanical ventilation in volumecontrolled mode with PEEP of 5 cm H2 O was provided adapting tidal volume and respiratory rate (fR) to target normocapnia (EtCO2 4.6-6 kPa) (35-45 mmHg) and a maximal inspiratory peak pressure of 20 cm H2 0. Heart rate (HR) and rhythm, fR, spirometry, pulse waves and saturation of oxygen (SpO2 ), invasive and non-invasive blood pressure (IBP and NIBP) measured with a 20 G cannulaj placed in the auricular artery and a cuff placed over the dorsal digital artery, respectively, esophageal temperature and inspired and expired gases (FiO2 , ExpO2 , FiCO2 , EtCO2 , EtIso) were continuously monitored and recorded every five minutes throughout anesthesiak .
aDormicum® 5 mg/ml, La Roche, CH
bMethadon Streuli® 10 mg/ml, Streuli Pharma AG, CH
During anaesthesia maintenance, a recruitment maneuver was successfully carried out to correct the relative hypoxemia (PaO2 of 15.2 kPa, (113.9 mmHg) with a FiO2 of 0.41) [1]. Maintenance was otherwise uneventful.
Two-hundred ten minutes after the induction of general anesthesia the sheep was transported to the recovery room where intermittent manual ventilation was provided with an AMBU bag connected to a source of oxygen until the sheep was able to breathe spontaneously and maintained normal EtCO2 . In the meanwhile, a nasal cannula was placed into the right nostril and secured to the skin. Monitoring during the recovery phase included pulse-oxymetry, capnography, inspired and expired O2 , T and IBPk . Extubation was performed when EtCO2 was ranging between 4.9 and 5.6 kPa (37 and 42 mmHg) and swallowing and active chewing were present. No signs of either regurgitation or aspiration were noticed. Instrumental monitoring was carried on via O2 and CO2 recording (left nostril), fR, IBP, and ECG; the values were continuously registered and recorded on papers every 15 minutes; at the same intervals, the neurological status was evaluated through a modified Glasgow coma scale (Table 1) adapted by Platt et al. [7]. Pain was evaluated every thirty minutes through a multi-parametric scale [8]; temperature, blood gases, glucose and electrolytes were evaluated at least every hourl .

Table 1: Modified Glasgow coma scale (adapted by Platt et al. [7]) used to evaluate the neurological status after intracranial surgery.
Oxygen (6-8 L minute-1) was supplemented through the nasal cannula and Plasma Lyte solution was infused at 2 mL kg-1h-1. Few minutes after tracheal extubation, the sheep was transferred to a purpose-made single cage and positioned on sternal recumbency. Blood pressure was: SAP 120 mmHg; DAP 73 mmHg; MAP 91 mmHg, (normal range: 116 ± 18; 74 ± 20; 91 ± 20) [9] HR was between 60-70 bpm-1 (normal range 70- 80 bpm-1) [10] and a blood gas analysis revealed hypoxemia (PaO2 =7.4 kPa, 55.5 mmHg) and hypercapnia (PaCO2 =7.75 kPa, 58.1 mmHg). Mild hypoxia was addressed through the placement of a second nasotracheal cannula through the contralateral nostril and connected to an oxygen source. After further twenty minutes the animal showed pinpoint pupils and a clonic seizure with sudden extensor rigidity of the four limbs and opisthotonus, followed by limb purposeless movements. After intravenous administration of diazepamm (0.2 mg kg-1), rigidity ameliorated but the sheep remained unresponsive and oculo-cephalic reflexes disappeared. Thereafter, after transient achycardia, blood pressure roseacutely; MAP approached 150 mmHg and sinus bradycardia (HR 42-44 beats minute-1) was concomitantly observed. Mannitol 20% (0.5 g kg-1)n was injected intravenously over ten minutes; severe tachypnea (fR 68) (normal range 12-20) with labored breathing pattern started shortly afterwards accompanied by loud respiratory noises and harsh, diffuse crackles could be auscultated on both sides of the thorax. A foamy blood tinged mild exudate appeared on the nostrils. A bolus of furosemideo (2 mg kg-1) was injected intravenously. Since in the ten following minutes respiratory pattern did not improve and hypoxaemia was recorded (PaO2 =7.3 kPa, 53.5 mmHg), a second bolus of furosemideo (2 mg kg-1) was injected. Thereafter, MAP decreased progressively and returned to baseline values over the following twenty minutes. Heart rate concomitantly increased; auscultation revealed improvement of the respiratory sounds, however, abdominal inspiratory effort was still noticed together with intermittent phases of tachypnea (fR between 32 and 42). Motor and cognitive functions improved progressively. Seventy five minutes after the onset of respiratory failure, the sheep stood up showing severe ataxia, appetite and spontaneous urination. Hundred minutes after standing, the alertness was deemed normal, HR was 80-84 beats minute-1, MAP was 112 mmHg (SAP 124 mmHg, DAP 94 mmHg), fR was 22 breaths minute-1and mild respiratory effort was intermittently recorded. With a FiO2 of 0.32, recorded PaO2 was 9.98 kPa (74.9 mmHg) at standing, 12.52 kPa (93.9 mmHg), 13.32 kPa (99.9 mmHg) and 11.32 kPa (84.9 mmHg), 12.85 kPa (96.4 mmHg) one, two, three and four hours after standing, respectively. Meloxicamp (0.5 mg kg-1) was administered intravenously a postoperative analgesia. Oxygen supplementation was continued overnight. Alaterolateral radiographic exam of the thorax obtained in standing position more than 12 hours after the acute episode revealed in the ventral lung field a patchy to confluent alveolar pattern with lack of visualization of vascular markings, and in the dorsal lung field a diffuse increased opacity with an ill-defined reticular background. The morning after the sheep was deemed clinically stable in light of improved lung auscultation, normal IBP, normal motor and cognitive function and appetite; an arterial blood gas at FiO2 of 0.21 revealed a PaO2 of 11.73 kPa (88 mmHg). The sheep was reintroduced in its herd under daily veterinary supervision.
cDomitor® 1 mg/ml , Provet AG, Lyssach, CH
d 8 Fr. 20 cm pediatric single-lumen Central Venous Catheterization Set, Arrow International Inc., PA, USA
eLidocain 2%®, Streuli Pharma AG, Uznach, CH f Propofol 1%® MCT Fresenius, Fresenius Kabi AG, CH
gAttane®, Isofluran 99.9%, Provet AG, Lyssach, CH
h
Fentanyl Curamed®i.v. 50 mg/ml, Actavis AG, CH
iPlasma-Lyte A, Baxter AG, Volketswil, CH
j20 G 32 mm Jelco 2 Intravenous Catheter Radiopaque, Smith MedicalInternational Ltd, Kent, UK
kMultiparametric monitor DatexOhmeda S5, GE, CH
lRAPIDPoint 500, Siemens Healthcare Diagnostics Ltd, Camberley, UK
mValium® Inj Lösung 10 mg/2ml, Roche Pharma AG, CH
nMannitol 20%, Bichsel AG, Interlaken, CH
oDimazon®, MSD Animal Health SARL, Lucerne, CH
Pulmonary complications are prevalent in critically-ill neurological patients. Their main causes are direct brain injury, depressed level of consciousness and inability to protect the airway, disruption of natural defense barriers and secondary physio pathologic insults inherent to severe brain injury. Due to numerous differential diagnoses and associated pathology, variable presentations and lack of specific test, NPE is a difficult diagnosis to be established nd straightforward diagnostic criteria are missing [11,12]. In fact, NPE is a diagnosis of exclusion and by definition requires documentation of non-cardiogenic pulmonary edema in the setting of neurological injury. In human beings, the early form of NPE is most common and is characterized by the development of symptoms within minutes to hours following neurologic injury. Epileptic seizures are reported among the causes of NPE [13-15]. The abrupt nature of respiratory distress is an impressive feature of NPE and typically the patient becomes acutely dyspneic, tachypnoeic and hypoxic within minutes. Pink, frothy sputum is commonly observed and bilateral crackles are appreciated on auscultation. Symptoms often spontaneously resolve within 24 to 48 hours, but NPE can persist longer in patients with elevated ICP [16,11].
In order to confirm NPE diagnosis, aspiration pneumonia must be ruled out. Regurgitation is a relative common feature in ruminants undergoing general anesthesia. In this case, neither regurgitation nor aspiration was witnessed during induction. Oropharyngeal and tracheal suctioning and flushing were performed as routine procedures before extubation, but gastric contents were not collected. Radiographically, neurogenic and other forms of non-cardiogenic pulmonary edema show typically a caudodorsal distribution in small animals and horses in the acute phase. However, it is known that the distribution may quickly change under effective treatment, thus, the available radiograph is not sufficient to evaluate the cause of the acute event. Although regurgitation could have passed unnoticed, an acute aspiration is unlikely to be linked to the sequence of the symptoms observed in this case. Indeed, the respiratory failure followed a seizure. Additionally, spontaneous clinical improvement without antibiotic therapy further supports the exclusion of aspiration pneumonia in this sheep. Other forms of subclinical preexisting pneumonia need to be taken into consideration, however in this casethe acuity of the observed symptoms deserves a further explanation.
pMetacam® 20 mg/ml, Boheringer Ingelheim GmBh, CH
Among other causes of non-cardiogenic pulmonary edema, negative pressure pulmonary edema (NPPE) needs to be considered. Spontaneous NPPE has been reported in human beings, in dogs, in horses and in sheep after tracheal obstruction [17]. Upper airway obstruction due to laryngospasm on emergence from anesthesiais the most common cause of NPPE in humans, but any intrinsic or extrinsic cause of airway obstruction can potentially lead to the same pathogenic cascade [18]. The sequel of events results in pulmonary edema within few minutes in human beings, but in horse pulmonary edema has been reported occurring within minutes to hours after the triggering event, so that the correlation between the causative event and the respiratory syndrome can be more challenging. In the sheep we reported here, extubation occurred smoothly and neither stridor, nasal edema norlack of air flow through the nostrils was noticed. Furthermore, tachypnea and crackles occurred suddenly after neurologic worsening and no inspiratory efforts were previously noticed. It is worth mentioning that after extubation arterial hypoxemia was recorded and therapeutically addressed.
In humanbeings ischemic-hypoxic encephalopathy after cardiac or respiratory arrest may cause seizures and transient hypoxia has been reported as cause of convulsive syncope, against which anticonvulsant medications are ineffective [19]. Whether a short term hypoxia could be linked to seizures in sheep is not known. However, in this case, neither respiratory nor cardiac arrest was noticed and the administration of diazepam was effective.
In horses, Ball and Trim [20] and Day et al. [21] hypothesized that intraanesthetic hypoxia may lead to hypoxic injury to capillary endothelium and alveolar epithelium and could result in pulmonary edema, whether short term post-anesthetic hypoxia could lead to severe pulmonary edema in sheep is not known, but cannot be completely ruled out.
There is strong evidence that alpha2 agonists induce a dose-dependent arterial hypoxemia in sheep whose degree is individual-dependent, and that increases when sympatholytic agents are administered concurrently [22-25]. Early changes in lung density and vessel diameters consistent with pulmonary congestion and edema formation were detected on CT scan after dexmedetomidine administration. However, in the same study, signs of regression on CT were already present at 30 minutes after administration of dexmedetomidine and capillary congestion on histology had resolved after thirty minutes. In the authors ‘opinion, it is likely that medetomidine administered in premedication in this sheep contributed to the suboptimal PaO2 recorded during anesthesia and represented a first hit on the pulmonary vascular changes. However, the authors consider less probable that medetomidine played a pivot role in the developing of the clinical acute post-operative pulmonary edema, in light of its time delaying.
The authors consider improbable that the pulmonary edema had a cardiogenic origin.
No cardiac abnormalities were clinically detected before anesthesia, inotropic support was not needed in any phase of either anesthesia or recovery and cardiac function was never impaired according to IBP. Fluid overload was unlikely since fluid management was overall rather conservative.
Taking all this into account, the authors would suggest that pulmonary edema observed in this sheep after implantation of deep brain electrodes was likely a NPE triggered by seizure. The potential abrupt increase in ICP would have led to neuronal compression, ischemia or damage that gave rise to an intense activation of the sympathetic nervous system and the release of catecholamines. The bradycardia appearing thereafter can be explained as baroreceptor reflex or as Cushing reflex. In experimental sheep, the increase in filtration and protein clearance in the pulmonary parenchyma occurred only after an increase in intracranial pressure that was large enough to elicit the Cushing response [5]. Despite the fact that the exact pathophysiology of NPE is still debated, a common denominator in all cases of NPE is a surge in endogenous serum catecholamines whose clinical manifestations may vary depending on the individual circumstance. While in some patients cardiac dysfunction predominates, in others capillary leakage is the primary manifestation. The different patterns have an obvious implication for the diagnosis and the treatment of individual cases [11]. To the best of the authors ‘knowledge, radiological features of NPE in sheep have never been described. In humans, bilateral pulmonary infiltrates and increased vascular shadowing are characteristic [5,11]. The management of NPE to date has largely focused on treating the underlying neurologic condition and osmotic diuretics, anti-epileptics and steroids have all been associated with improvements in oxygenation [13,26]. The use of an α-blocking agent, as phentolamine, could be considered since in experimental sheep its administration prevented the increase in pulmonary permeability after neurologic insult [5]; however no clinical evidence of its benefit has been reported so far.
In conclusion, neurogenic pulmonary edema should be taken into consideration as potential complication following intraparenchymal cerebral surgeries in sheep. In our case, anti-epileptics and diuretics were successful in overcoming the respiratory failure. In an experimental setting, pulmonary artery catheterization could offer useful pathogenic information; indeed, the role of elevation of systemic and pulmonary pressure in development of NPE has not completely elucidated yet.
The Authors would like to thank Dr.Med.Vet. Daniel Mettler for his precious contribution during the intraoperative management of the sheep and Prof. Claudia Spadavecchia for her continued scientific and human support.
The Authors declare no conflict of interest. The intensive care of the sheep was funded by AlevaNeurotherapeutics SA, EPFL Innovation Park, Building D, 1015 Lausanne, Switzerland.
Download Provisional pdf here
All Sci Forschen Journals are Open Access