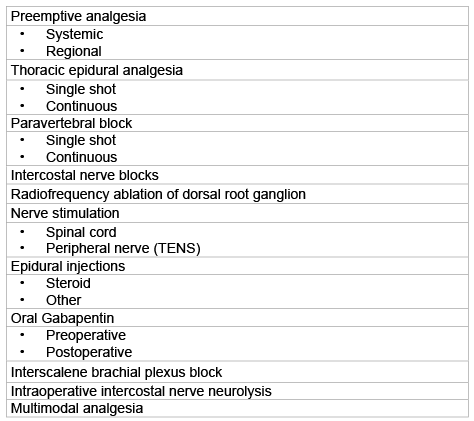
Table 1: Treatment options for post-thoracotomy pain.

Baturay Kansu Kazbek1 Ahmet Gokhan Gundogdu2 Tevfik Kaplan3 Serdar Han3 Gultekin Gulbahar2*
1Department of Anesthesiology and Reanimation, Ufuk University, School of Medicine, Ankara, Turkey*Corresponding author:Gultekin Gulbahar, Division of Thoracic Surgery, Dr. Nafiz Korez Sincan State Hospital, Ankara, Turkey, Tel: 90 505 3359531; Fax: 90 312 2735151; E-mail: mdgultekin@ gmail.com
Aritcle Type: Review Article
Citation: Kazbek BK, Gundogdu AG, Kaplan T, Kocer B, Han S, et al. (2016 Post-Thoracotomy Paın Management. J Clin Anesth Manag 1(2): doi http:// dx.doi.org/10.16966/2470-9956.105
Copyright: © 2016 Kazbek BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Thoracotomy is a very painful procedure which may be performed for a bunch of reasons. Pain following thoracotomy incision is multifactorial and difficult to treat. Treatment of post-thoracotomy pain not only provides patient comfort but also prevents postoperative pulmonary morbidity. Chronic post-thoracotomy pain has a serious negative impact on the quality of life. Although thoracic epidural analgesia has been traditionally accepted as the gold standard, paravertebral block has been gaining popularity in recent time. This review attempts to investigate the treatment options for post-thoracotomy pain.
Thoracotomy; Pain management; Analgesia; Anesthesia; Regional; Treatment efficacy
Thoracotomy is one of the surgical procedures most associated with severe postoperative pain. Postoperative pain can cause severe respiratory problems such as hypoxia, atelectasis and pulmonary infections. These conditions may result in dreadful situations and lead to respiratory failure [1]. Therefore, pain management is of crucial importance to minimize morbidity and mortality rates in patients who have undergone thoracotomy.
Pre-emptive analgesia has been proposed as an effective method of pain control. Although there are arguments over the effectiveness of this method, both systemic and regional analgesia have been used for the prevention of postthoracotomy pain. Clonidine, corticosteroids, nonsteroid antiinflammatory drugs, ketamine and pregabalin have been used as agents. Tschenko et al. [2] used pre-emptive clonidine (single dose intramuscular injection, 2 mcg/kg) and reported that it did not decrease Visual Analog Scale (VAS) scores or analgesic requirements compared to placebo. Bigler et al. [3] compared a single 25 mg/kg intravenous injection of methylprednisolone to placebo on 38 patients and reported that pre-emptive corticosteroid bolus reduced VAS scores after 4 hours and on the first day at rest and after 4 and 8 hours on the second day during cough. Application of steroids did not have a positive effect on pulmonary functions. Moreover, three patients in the corticosteroid group had to undergo reoperation. The authors stated that preoperative steroid injection is not warranted.
Ketamine, which is an N-Methyl-D-Aspartate (NMDA) receptor antagonist, has been used for pre-emptive analgesia by Ozyalçın et al. [4] in a study conducted in 2004. In this study, sixty patients were divided into 3 groups (intramuscular ketamine 1 mg/kg plus epidural saline, epidural ketamine 1 mg/kg plus intramuscular saline and finally intramuscular and epidural saline). The authors stated that postoperative epidural morphine and local anesthetic consumption was lower in the epidural ketamine group. The study does not report any side effecs attributable to epidural ketamine usage.
Kinney et al. [5] compared gabapentin/pregabalin as pre-emptive analgesia for post-thoracotomy pain in 2012. The patients undergoing thoracotomy received 600 mg gabapentin 2 hours before the operation and were given continous epidural infusion of gabapentin combined with ketorolac and acetaminophen. The authors stated that pre-emptive gabapentin did not reduce pain scores or opioid consumption following elective thoracotomy.
Although the efficacy of pre-emptive analgesia has been subject to debate, pre-emptive analgesia is believed to decrease the incidence of postoperative pain syndromes by changing the central processing of painful stimuli [6-9]. Pre-emptive epidural local anesthetic with or without opioids have also been used for the management of pain following thoracotomy. This method has been traditionally compared to systemic analgesia. Pre-emptive thoracal treatments offer the advantage of avoiding common postoperative side effects often associated with systemic analgesic treatment such as nausea, vomiting, sedation and difficulty in ambulation in the early postoperative period. However, regional blocks have their own morbidities. For example, pre-emptive single dose bolus of intrathecal opioids have been shown to increase the incidence of urinary retention despite the fact that they reduce the pain scores in the postoperative period [10]. Numerous studies, which compare different forms of regional pre-emptive analgesia to systemic analgesia, have been carried out. These studies have compared thoracic epidural, thoracic paravertebral, intercostal, and intrapleural blocks to systemic analgesia.
Pre-emptive thoracal epidural applications have been extensively reviewed. Thoracic epidural analgesia (TEA) is considered as the gold standard [11]. It has also been shown to be superior to conventional analgesia models in postthoracotomy pain [12,13]. In a study conducted by Ertürk et al. [14] co-infusion of levobupivacaine and fentanyl following initiating bolus injection was compared to that of saline. It was reported that local anesthetic and opioid combination decreased VAS scores at rest and coughing state. The authors did not report any differences between side effects. In a similar fashion, Yegin et al. [15] compared preoperative versus postoperative initiation of TEA and reported that pre-emptive application of thoracic analgesia significantly lowers pain scores. Although possible benefits and harms of TEA are subject to debate, it is decidedly superior to intravenous PCA application. Nausea, vomiting, possible motor block, hypotension and consequent cardiovascular problems and inadequate control of pain can be numbered among disadvantages of this technique.
Although a considerable number of studies report successful decrease in pain scores by pre-emptive analgesia yet [16-18], there are other studies which state that pre-emptive analgesia is of little or no clinical value [19- 22]. Effective pre-emptive analgesia requires a total afferent block which lasts for 2 or 3 days in addition to blockade of circulating pro-inflammatory cytokines (i.e. IL-1β), which is difficult to achieve in vivo [23].
Surgical stress causes humoral mediator release. In a study by Amr et al. [24] the researchers investigated the effects of preoperative epidural local anesthetic application on respiratory and endocrine systems. Although significant improvement was observed in respiratory functions and analgesia in the intervention group; there was no statistically significant improvement in oxygenation, cortisol or glucose release levels between the two groups. This could be due to relatively low dose of bupivacaine used in the study. This seems to be caused by the desire to avoid motor blockade in the postoperative period. Although pain scores were lower, VAS scores on coughing state were higher in the intervention group in the early postoperative period. Choosing a different local anesthetic with a lower tendency to cause motor blockade could have yielded different results.
In regional anesthesia, adjuvants to local anesthetics are widely used. However, there are studies which suggest that their use in TEA does not enhance the quality of analgesia [25-27]. Blanloeil et al. [25] reported that addition of methylprednisolone to continuous epidural infusion of local anesthetics does not cause any difference in postoperative pain scores or morphine requirements. On the other hand, a recent study compared the effects of magnesium sulphate and clonidine and reported that the addition of clonidine provided a longer analgesia, followed by magnesium sulphate. Clonidine also reduced the number of additional analgesics but it also increased the incidence of sedation [28]. Similarly, a study conducted by Chia et al. [29] compared the effects of pre-emptive and intraoperative epidural neostigmine. It is reported that pre-emptive continuous neostigmine decreases epidural analgesic consumption in the first six postoperative days.
Although TEA is accepted as the gold standard for the management of post-thoracotomy pain, it may be contraindicated in some patients. Additionally, it may be associated with complications such as hypotension, urinary retention, unsuccessful blocks and neurological sequelae. Paravertebral block (PVB) has emerged as an alternative to TEA. A recent study by Dango et al. [30] reports a statistically significant but yet small and questionable decrease in VAS scores when thoracic epidural anesthesia and a combination of PVB and intrathecal opioid are used. This combination is thus suggested as a viable alternative to thoracic epidural blocks. Pintaric et al. [31] compared continuous thoracic epidural analgesia to PVB and reported that the two technics provide similar analgesia, but the patients in the PVB group require less colloids and vasopressors to maintain the target oxygen delivery index. Scarci et al. [32] investigated more than 184 papers for comparison of thoracic paravertebral and epidural blocks. They stated that in PVB groups, VAS scores at rest and coughing state are significantly lower; oximetry measurements and respiratory function test scores are better. In epidural analgesia groups, adverse effects such as urinary retention, nausea, itching and hypotension are higher and technical difficulties are more frequent. Although plasma concentrations of cortisol are elevated in both groups, this elevation is less pronounced in the PVB group. Similarly, a systematic review by Davies et al. [1] evaluated randomized controlled trials concerning studies which compared PVB to TEA in thoracic surgery. According to the results of ten studies, although there was no statistically significant difference between pain scores in the postoperative period; pulmonary and systemic complications were more frequent in the thoracic epidural group. Moreover, technical difficulties were more commonly met in the thoracic epidural group. As a result, they recommended PVB for thoracic surgery. Richardson et al. [33] also compared PVB and TEA. Better pulmonary function test results, lower pulmonary morbidity and better oxygenation levels were observed in PVB group. In TEA group, 10 patients were omitted from the study due to the failure in epidural catheter insertion. PVB is a relatively new technique compared to TEA. Although there is no certain evidence pointing to its superiority in pain control over TEA, its lack of disadvantages attributed to TEA and possibility of catheterisation under direct visualization in the intraoperative period by the surgeon can be considered as an advantage of this technique.
Catheterization for PVB can be achieved either percutaneously before the surgery or during the operation before the closure of the thoracic cage. Still, some probable risks exist for preoperative percutaneous catheterization. In a series of 367 patients, Lönquist et al. [34] sought for PVB related clinical problems. They reported hypotension, vascular puncture and pneumothorax as possible complications. Several studies emphasize the safety of placing the catheter under direct vision for the prevention of various complications [33-37]. A randomized trial by Gulbahar et al. [38] compares the effectiveness of TEA and PVB on postoperative pain control. They report that although VAS scores, pulmonary function tests and serum cortisol levels are similar between the two groups, PVB group is associated with shorter intervention time and less iatrogenic complications in comparison of epidural and paravertebral catheterisation techniques in post-thoracotomy pain management. A recent study failed to show any differences in efficacy between bolus and controlled infusion of local anesthetics via elastomeric pumps through a catheter placed under direct visualization [39].
The commonly accepted definition of postthoracotomy pain syndrome (PTPS) is pain that recurs or persists along a thoracotomy incision at least two months following the surgical procedure [40]. It shares the characteristics of neuropathic pain (such as burning, stabbing accompanied by dysesthesia). According to Perttunen et al. [41] the incidence of long-term postthoracotomy (Table 1) pain is 80% at three months, 75% at six months and 61% at one year and nearly half of the patients suffer from debilitating pain. Moreover, patients who suffer from PTPS report unsatisfactory results from various and costly therapies [42].

Table 1: Treatment options for post-thoracotomy pain.
Proposed mechanisms for the occurence of chronic postthracotomy pain are diverse and include psychological factors [43], recurrence of the primary tumor [44], incision type [45] and direct damage to intercostal nerves [46]. The observation of allodynia and hyperalgesia in the areas innervated by the intercostal nerves suggests that the nerve injury caused by retraction or the incision itself is a likely culprit for chronic postthoracotomy pain.
Sapkota et al. [47] hypothesized that intercostal nerve injury is the main reason for postthoracotomy pain. They compared pericostal sutures technics and found that suture technic with an intercostal muscle flap which is harvested at the beginning of the operation lowered postoperative pain scores without increasing total operation time. Likewise, Leandro et al. [48] compared pericostal and transcostal sutures. They found that transcostal sutures yield lower early and late pain scores. Elshiekh et al. [49] report that muscle-sparing thoracotomy confers a one-month advantage in skeletal muscle strength and range of motion over classical posterolateral thoracotomy
TEA appears to be effective at reducing the incidence of PTPS however the time of initiation does not seem to be a determinant of this incidence [50]. According to a meta-analysis published by Bong et al. [51] who investigated six studies and 458 patients; although pre-emptive thoracic anesthesia provides a significant reduction in pain scores on coughing at 24 and 48 hours, there is no significant difference concerning the incidence of chronic pain at 6 months between pre-emptive TEA (39.6%) and control groups (48.6%). The effectiveness of PVB has not been fully investigated in prevention of PTPS. Intercostal nerve blocks, local anesthetic injections and cryoanalgesia do not seem to be effective. Moreover, cryoanalgesia may increase the incidence of neuropathic symptoms [52]. Ketamine has been investigated in two randomized studies and did not decrease the incidence of PTPS [53,54]. Mac et al. [55] report that pre-emptive and 48-hour acetamibophen treatment decreases ipsilateral shoulder pain incidence following thoracotomy.
Since PTPS has a neuropathic component, gabapentin may potentially treat this clinical condition. Solak et al. [56] compared gabapentin to naproxen sodium and showed gabapentin to be effective. Invasive interventions such as nerve blocks, radiofrequency of dorsal root ganglion, epidural injections, spinal cord and peripheral nerve stimulation may also be used.
In summary, effective postoperative pain control and treatment after thoracotomy, which is one of the most painful surgical procedures, is crucial in order to avoid pulmonary complications, delayed mobilization, late discharge and chronic pain. Although local anesthetics and opioids given via thoracic epidurals have long been acepted as gold standard for this purpose, paravertebral blocks are gaining popularity and offer the same level pain control with less complications.
Both thoracic surgeons and pain specialists should review the treatment options and tailor the chosen treatment modality for every patient’s unique requirements while taking patient’s decisions and other medical conditions into consideration.
Download Provisional pdf here
All Sci Forschen Journals are Open Access